Is Water Freezing Exothermic Or Endothermic
listenit
Mar 21, 2025 · 5 min read
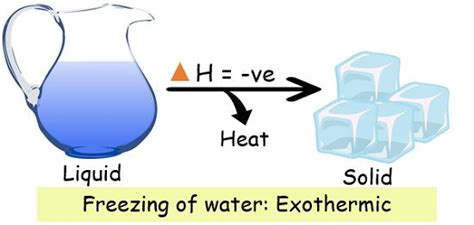
Table of Contents
Is Water Freezing Exothermic or Endothermic? Understanding Phase Transitions
The question of whether water freezing is exothermic or endothermic often trips up students and even seasoned scientists if they aren't careful with their definitions. While seemingly simple, understanding this process delves into the fundamental principles of thermodynamics and the behavior of matter at a molecular level. This comprehensive guide will explore the intricacies of water freezing, clarifying the energy transfers involved and dispelling common misconceptions.
Defining Exothermic and Endothermic Processes
Before we delve into the specifics of water freezing, let's establish a clear understanding of the core terminology:
Exothermic Process: An exothermic process releases energy into its surroundings. This energy is often released as heat, causing a temperature increase in the environment. The system's energy decreases, while the surroundings' energy increases. Think of burning wood – it releases heat, making it an exothermic reaction.
Endothermic Process: An endothermic process absorbs energy from its surroundings. This absorption of energy often manifests as a decrease in the temperature of the environment. The system's energy increases while the surroundings' energy decreases. Melting ice is a classic example of an endothermic process; it absorbs heat from the surroundings to change from solid to liquid.
The Freezing of Water: A Detailed Look
Water freezing is the transition from the liquid (water) phase to the solid (ice) phase. This phase transition is driven by the decrease in temperature. But the crucial point is what happens to the energy during this transformation.
Molecular Perspective
In liquid water, water molecules are relatively mobile, constantly moving and interacting with each other through hydrogen bonds. These bonds are relatively weak, allowing for fluidity. As the temperature decreases, the kinetic energy of the molecules decreases. This reduced kinetic energy means the molecules move slower.
At 0°C (32°F) at standard atmospheric pressure, the kinetic energy of the water molecules becomes low enough that the attractive forces between them (hydrogen bonds) become dominant. The molecules begin to arrange themselves into a highly ordered crystalline structure – ice. This ordered structure is less energetically favorable for the individual molecules, and the process of forming this structure requires energy to be released.
Energy Transfer During Freezing
This is where the key understanding lies. The process of forming the ordered crystalline structure of ice involves the release of energy. This energy is released to the surroundings, leading to a slight increase in the temperature of the immediate environment. This is why the freezing of water is an exothermic process.
The confusion often arises from focusing solely on the temperature change of the water itself. The temperature of the water remains at 0°C during the freezing process until all the water has solidified. However, this constant temperature doesn't negate the energy release. The energy is being released to the surroundings, not used to further lower the temperature of the water. This distinction is crucial.
Misconceptions and Clarifications
Several misconceptions surround the exothermic nature of water freezing. Let's address some of the most common:
-
The temperature of the water decreases: While the temperature of the water initially decreases before reaching the freezing point, during the freezing process the temperature remains constant at 0°C. This constant temperature is a hallmark of a phase transition. The energy is being released, not used to further cool the water.
-
It feels cold: The fact that freezing water feels cold is often misinterpreted. This coldness is due to the transfer of heat from your hand to the water, as the water absorbs heat from its surroundings to maintain the phase transition. It's not the water releasing cold; it's the water absorbing heat from warmer surroundings, causing a cooling sensation.
-
Confusing freezing with cooling: Cooling is a process of lowering the temperature. Freezing is a phase transition at a constant temperature. While cooling leads to freezing, the two are distinct processes. The cooling process is exothermic (energy is released from the water as it cools), but the freezing process itself, the phase change, is also exothermic due to the release of energy when molecules crystallize.
Real-World Applications and Implications
The exothermic nature of water freezing has several significant real-world implications:
-
Weather and Climate: The release of energy during the freezing of water plays a crucial role in moderating weather patterns and climate. The freezing of large bodies of water releases substantial amounts of heat into the atmosphere.
-
Ice Formation: The exothermic nature of freezing is crucial in understanding how ice forms and its impact on ecosystems.
-
Industrial Processes: Many industrial processes leverage the exothermic nature of freezing for various applications like food preservation, ice production, and even certain chemical reactions.
Beyond Water: Phase Transitions in Other Substances
It is important to note that the exothermic nature of freezing is not unique to water. Most substances release heat when they transition from liquid to solid. However, the specific amount of heat released (latent heat of fusion) varies significantly depending on the substance's intermolecular forces and crystal structure.
Conclusion: Embracing the Exothermic Nature of Freezing
Water freezing is an exothermic process because the formation of the ordered crystalline structure of ice releases energy into the surroundings. It is crucial to distinguish between the constant temperature during the freezing process and the overall energy release, understanding that the energy is released to the surroundings, not used to lower the temperature further. This seemingly simple concept underlies numerous natural processes and technological applications, highlighting the importance of understanding phase transitions and thermodynamics. By clarifying this seemingly simple concept, we gain a deeper appreciation of the intricate processes governing the world around us. This knowledge is key to understanding various natural phenomena and developing new technologies.
Latest Posts
Latest Posts
-
X 3 X 2 X Factor
Mar 28, 2025
-
4 To The Power Of Negative 1
Mar 28, 2025
-
What Is A Common Property Of Metals
Mar 28, 2025
-
What Does The Coefficient In A Chemical Equation Represent
Mar 28, 2025
-
1 4 Pound Is How Many Ounces
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is Water Freezing Exothermic Or Endothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
