Is Sugar A Compound Element Or Mixture
listenit
Mar 18, 2025 · 5 min read
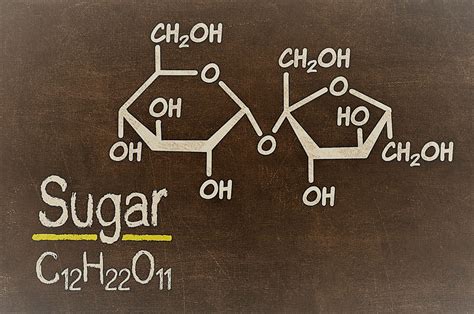
Table of Contents
Is Sugar a Compound, an Element, or a Mixture? Understanding the Chemistry of Sweetness
Sugar, a ubiquitous component of our diet and a cornerstone of countless culinary creations, often sparks curiosity about its fundamental nature. Is it an element, a compound, or a mixture? This comprehensive exploration delves into the chemical composition of sugar, differentiating it from elements and mixtures and clarifying its classification within the realm of chemistry.
Understanding the Basic Chemical Classifications
Before diving into the specifics of sugar, let's establish a clear understanding of the three primary classifications of matter: elements, compounds, and mixtures.
Elements: The Building Blocks of Matter
Elements are the fundamental substances that cannot be broken down into simpler substances by chemical means. They are composed of only one type of atom, identified by its unique atomic number (the number of protons in its nucleus). Examples include hydrogen (H), oxygen (O), carbon (C), and iron (Fe). Elements are listed on the periodic table.
Compounds: Elements Bound Together
Compounds are substances formed when two or more elements chemically combine in fixed proportions. This combination involves a chemical reaction, resulting in a new substance with properties distinct from its constituent elements. The elements in a compound are held together by chemical bonds, typically covalent or ionic bonds. Water (H₂O), salt (NaCl), and carbon dioxide (CO₂) are classic examples of compounds. The properties of a compound are dramatically different from the properties of its constituent elements. For instance, hydrogen and oxygen are gases, but their combination forms liquid water.
Mixtures: A Blend of Substances
Mixtures are physical combinations of two or more substances that are not chemically bonded. The components retain their individual properties, and their proportions can vary. Mixtures can be homogeneous (uniform composition throughout, like saltwater) or heterogeneous (non-uniform composition, like sand and water). Crucially, mixtures can be separated into their components by physical methods, such as filtration or distillation. Unlike compounds, no chemical reaction occurs when substances are mixed.
The Chemical Structure of Sugar: A Deep Dive
Now, let's focus on sugar. The term "sugar" encompasses a broad class of carbohydrates, but the most common type is sucrose, also known as table sugar. Sucrose's chemical formula is C₁₂H₂₂O₁₁. This formula immediately reveals that sucrose is not an element. Elements have only one type of atom; sucrose is clearly made up of three different elements: carbon, hydrogen, and oxygen.
Is Sucrose a Compound or a Mixture?
Because sucrose is composed of multiple elements (carbon, hydrogen, and oxygen) chemically bonded together in a fixed ratio (12:22:11), it is unequivocally classified as a compound, not a mixture. The atoms are not simply mixed together; they are interconnected through strong covalent bonds, forming a complex molecule with unique properties. You can't separate sucrose into carbon, hydrogen, and oxygen using simple physical methods; you need a chemical reaction (like combustion).
Different Types of Sugars: Still Compounds
While sucrose is the most common table sugar, other sugars exist, including:
- Glucose (C₆H₁₂O₆): Often called "blood sugar," glucose is a simple sugar and a crucial source of energy for living organisms.
- Fructose (C₆H₁₂O₆): Found naturally in fruits, fructose is another simple sugar, often sweeter than glucose.
- Lactose (C₁₂H₂₂O₁₁): The sugar found in milk, lactose is a disaccharide composed of glucose and galactose.
All of these sugars are compounds, not mixtures or elements. They all consist of carbon, hydrogen, and oxygen atoms chemically bound in specific ratios, defining their unique properties. Even though some, like lactose, are composed of simpler sugar units, the linkage is a chemical bond forming a distinct compound.
Differentiating Sugar from Mixtures
Let's contrast sugar with some potential mixtures to further solidify its classification as a compound.
Sugar Water: A Homogeneous Mixture
Dissolving sugar in water creates a homogeneous mixture. The sugar molecules are dispersed throughout the water, but no chemical reaction occurs. The sugar and water retain their individual properties; you can easily separate them through evaporation (leaving the sugar behind).
Sand and Sugar: A Heterogeneous Mixture
A mixture of sand and sugar is heterogeneous. The sand and sugar particles are physically mixed but not chemically bonded. You can separate them using physical methods such as sifting or dissolving the sugar in water and filtering out the sand.
The Key Difference: Chemical Bonding vs. Physical Mixing
The critical difference between a compound (like sugar) and a mixture (like sugar water or sand and sugar) lies in the presence or absence of chemical bonds. In compounds, the elements are chemically bonded, forming a new substance with unique properties. In mixtures, the components are physically combined, retaining their individual characteristics.
The Importance of Understanding Sugar's Chemical Classification
Understanding that sugar is a compound, not a mixture or an element, has important implications in various fields:
- Nutrition and Health: The chemical structure of sugar dictates its metabolism in the body, impacting energy levels and overall health.
- Food Science and Technology: The properties of sugar—its sweetness, solubility, and reactivity—are crucial for food processing and preservation.
- Chemistry and Biochemistry: Sugar serves as a model compound for understanding the principles of organic chemistry and the role of carbohydrates in biological systems.
Conclusion: Sugar's Chemical Identity
In summary, sugar, specifically sucrose and other related carbohydrates, is a compound. It's composed of carbon, hydrogen, and oxygen atoms chemically bonded together in a fixed ratio. This chemical bonding differentiates it from both elements and mixtures. Understanding this fundamental classification is essential for comprehending the role of sugar in various contexts, from nutrition and health to food science and chemistry. The distinct properties of sugar arise directly from its chemical structure and the strong covalent bonds holding its constituent atoms together. Therefore, the next time you enjoy a sweet treat, remember the fascinating chemistry involved in the compound that makes it so delicious.
Latest Posts
Latest Posts
-
Which Of The Following Elements Is A Transition Metal
Mar 19, 2025
-
Atoms Of The Same Element Have The Same Number Of
Mar 19, 2025
-
Can A Element Be Broken Down
Mar 19, 2025
-
Calculating The Ph At The Equivalence Point
Mar 19, 2025
-
What Is The Equivalent Fraction For 3 4
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Sugar A Compound Element Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
