Which Of The Following Elements Is A Transition Metal
listenit
Mar 19, 2025 · 6 min read
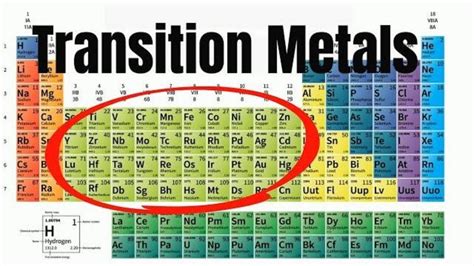
Table of Contents
Which of the Following Elements is a Transition Metal? A Comprehensive Guide
Transition metals are a fascinating group of elements that form the bridge between the highly reactive alkali and alkaline earth metals and the less reactive, more diverse p-block elements. Understanding what defines a transition metal is crucial in chemistry, as their unique properties lead to a wide array of applications in various fields. This comprehensive guide will explore the defining characteristics of transition metals, delve into the reasons behind their properties, and ultimately, help you confidently identify them.
Defining Transition Metals: Beyond the Periodic Table
The simple answer to "which of the following elements is a transition metal?" relies on understanding their location within the periodic table. Transition metals are found in the d-block of the periodic table, specifically groups 3-12. However, the definition extends beyond this simple placement. Several key properties characterize these elements:
1. Partially Filled d-Orbitals: The Core Characteristic
The most fundamental characteristic of a transition metal is the presence of a partially filled d-orbital in at least one of its oxidation states. This partially filled d-orbital is responsible for many of the unique properties displayed by transition metals, including variable oxidation states, colorful compounds, and catalytic activity.
Unlike main group elements which primarily utilize s and p orbitals in bonding, transition metals readily involve their d-orbitals, leading to a wider range of bonding possibilities and complex formation.
2. Variable Oxidation States: A Hallmark of Transition Metals
Transition metals exhibit a remarkable ability to exist in multiple oxidation states. This means a single transition metal atom can lose a varying number of electrons to form different ions. For example, iron (Fe) can exist as Fe²⁺ (ferrous) and Fe³⁺ (ferric), contributing to the diverse chemistry of iron compounds. This variability arises from the relatively small energy difference between the (n-1)d and ns orbitals, making it energetically favorable for electrons to be lost from either.
This property significantly impacts the reactivity and the types of compounds formed by transition metals, leading to a vast array of applications, from pigments to catalysts.
3. Formation of Colored Compounds: The Spectacle of d-Electron Transitions
The colorful nature of many transition metal compounds is another striking characteristic. This color arises from the d-d electronic transitions. When a transition metal ion absorbs light, electrons within the partially filled d-orbitals can be excited to higher energy levels. The specific wavelengths of light absorbed depend on the electronic configuration of the ion and the ligands (surrounding atoms or molecules) coordinated to it. The light that is not absorbed is transmitted or reflected, giving the compound its characteristic color.
The intensity and wavelength of absorbed light are influenced by various factors, including the oxidation state of the metal, the nature of the ligands, and the geometry of the complex. This makes the color of transition metal compounds a valuable tool for identifying and characterizing these substances.
4. Catalytic Activity: Facilitating Chemical Reactions
Transition metals and their compounds are renowned for their catalytic activity. This stems from their ability to readily change their oxidation states and form temporary bonds with reactant molecules, lowering the activation energy required for a reaction to proceed. This makes them indispensable in countless industrial processes, including:
- Haber-Bosch process: Production of ammonia (NH₃) from nitrogen (N₂) and hydrogen (H₂), crucial for fertilizers.
- Ziegler-Natta polymerization: Production of plastics such as polyethylene and polypropylene.
- Automotive catalytic converters: Converting harmful exhaust gases into less harmful substances.
The ability to act as catalysts stems directly from their variable oxidation states and the availability of d-orbitals for bonding interactions.
5. Magnetic Properties: A Result of Unpaired Electrons
Many transition metal compounds exhibit magnetic properties due to the presence of unpaired electrons in their d-orbitals. These unpaired electrons create magnetic moments that interact with external magnetic fields. This property is exploited in various applications, such as magnetic recording media and magnetic resonance imaging (MRI).
The strength and type of magnetism (paramagnetism, ferromagnetism, etc.) depend on the electronic configuration of the metal ion and the interactions between neighboring ions in the solid state.
6. High Melting and Boiling Points: Strong Metallic Bonding
Transition metals generally have high melting and boiling points compared to main group elements. This is attributed to the strong metallic bonding arising from the delocalized electrons in the d-orbitals and the s-orbitals. These delocalized electrons contribute to the strong cohesive forces within the metallic lattice.
Identifying Transition Metals: Practical Applications
Now, let's apply this knowledge to identify transition metals from a given list. Consider the following elements:
- Sodium (Na): Alkali metal, group 1
- Iron (Fe): Transition metal, group 8
- Oxygen (O): Nonmetal, group 16
- Copper (Cu): Transition metal, group 11
- Chlorine (Cl): Halogen, group 17
- Titanium (Ti): Transition metal, group 4
- Zinc (Zn): Transition metal, group 12
- Gold (Au): Transition metal, group 11
- Argon (Ar): Noble gas, group 18
- Manganese (Mn): Transition metal, group 7
Based on the criteria above, the transition metals in this list are: Iron (Fe), Copper (Cu), Titanium (Ti), Zinc (Zn), Gold (Au), and Manganese (Mn). They all reside in the d-block of the periodic table and exhibit the characteristic properties discussed earlier.
Exceptions and Borderline Cases
While the d-block elements are generally considered transition metals, there are a few exceptions and borderline cases that warrant clarification:
-
Zinc (Zn), Cadmium (Cd), and Mercury (Hg): These elements in group 12 have completely filled d-orbitals in their ground state. However, they are traditionally included with the transition metals due to their similar chemical properties, such as exhibiting multiple oxidation states (although fewer than other transition metals) and forming coordination complexes.
-
Scandium (Sc) and Yttrium (Y): While technically in the d-block, their chemistry often resembles that of the alkaline earth metals more closely, leading to some debate on their classification.
These borderline cases highlight the complexities involved in defining and classifying chemical elements, but ultimately, their inclusion amongst the transition metals reflects the overarching similarities in their chemical behavior.
The Importance of Transition Metals in Everyday Life
The unique properties of transition metals make them vital to our modern society. Their applications span diverse fields:
-
Construction: Steel (an alloy of iron and carbon) is a ubiquitous construction material. Other transition metals like titanium are used in high-strength, lightweight alloys for aerospace applications.
-
Electronics: Copper is an excellent conductor of electricity and widely used in wiring and electronics. Other transition metals are employed in various electronic components.
-
Medicine: Platinum-based drugs are used in cancer chemotherapy. Other transition metals play roles in biological systems as essential trace elements.
-
Catalysis: Transition metal catalysts are essential in numerous industrial processes, such as petroleum refining and the production of chemicals.
-
Pigments and Dyes: Transition metal compounds are used extensively as pigments and dyes in paints, fabrics, and cosmetics. Their vibrant colors are a direct result of their d-electron transitions.
Conclusion: Mastering the Identification of Transition Metals
Identifying transition metals requires a nuanced understanding of their defining characteristics, going beyond simple periodic table placement. The partially filled d-orbitals, variable oxidation states, color formation, catalytic activity, magnetic properties, and high melting/boiling points all contribute to the unique nature of these elements. By understanding these properties, you can confidently identify transition metals and appreciate their crucial role in various aspects of our lives. The ability to discern these elements from other groups within the periodic table is an essential skill for any aspiring chemist or anyone fascinated by the wonder of the elements.
Latest Posts
Latest Posts
-
Atoms Of The Same Element That Have Different Masses
Mar 19, 2025
-
66 And 2 3 As A Fraction
Mar 19, 2025
-
How Are Positive And Negative Ions Formed
Mar 19, 2025
-
How Many Ounces Is 500 Ml Of Water
Mar 19, 2025
-
Revolutions Per Second To Radians Per Second
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Elements Is A Transition Metal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
