Atoms Of The Same Element That Have Different Masses
listenit
Mar 19, 2025 · 6 min read
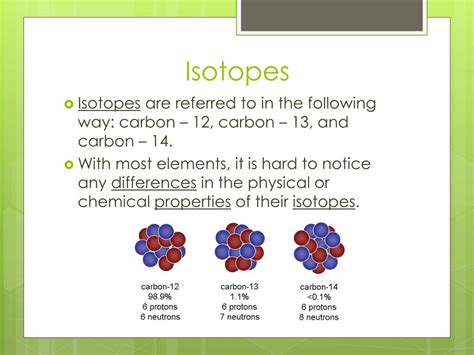
Table of Contents
Atoms of the Same Element That Have Different Masses: Isotopes Explained
Atoms are the fundamental building blocks of matter, but their simplicity belies a fascinating complexity. While we often learn that atoms of the same element are identical, this isn't entirely accurate. Atoms of the same element can indeed have different masses, a phenomenon known as isotopes. Understanding isotopes is crucial to grasping the nuances of chemistry, nuclear physics, and even geological dating. This article will delve deep into the world of isotopes, exploring their properties, applications, and significance.
What are Isotopes?
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. Recall that the atomic number of an element is defined by the number of protons in its nucleus. This number determines the element's identity and its position on the periodic table. However, the number of neutrons can vary, leading to different isotopes of the same element.
For example: Consider carbon (C), with an atomic number of 6. This means every carbon atom has 6 protons. However, carbon has several isotopes:
- Carbon-12 (¹²C): 6 protons and 6 neutrons. This is the most common and stable isotope of carbon.
- Carbon-13 (¹³C): 6 protons and 7 neutrons. This is also a stable isotope, though less abundant than ¹²C.
- Carbon-14 (¹⁴C): 6 protons and 8 neutrons. This is a radioactive isotope with a half-life of approximately 5,730 years. Its radioactivity is used in carbon dating.
The number following the element's name represents the mass number, which is the sum of protons and neutrons in the nucleus. Therefore, isotopes of the same element have the same atomic number but different mass numbers.
Notation and Representation of Isotopes
Isotopes are typically represented using different notations:
- Nuclear notation: This notation uses the element's symbol, with the mass number as a superscript to the left and the atomic number as a subscript to the left. For example, ¹²₆C represents carbon-12.
- Hyphen notation: This notation simply uses the element's name followed by the mass number, such as carbon-12 or carbon-14.
Both notations are widely used, and the context usually makes it clear which notation is being employed.
Properties of Isotopes
While isotopes of the same element have the same number of protons and electrons, their different neutron numbers impact several properties:
- Mass: Isotopes have different masses due to the differing number of neutrons. This mass difference is significant in some applications, such as mass spectrometry.
- Nuclear stability: Some isotopes are stable, meaning their nuclei do not spontaneously decay. Others are radioactive, meaning their nuclei are unstable and undergo radioactive decay, emitting particles or energy to become more stable. The stability of an isotope depends on the balance between the strong nuclear force (which holds the nucleus together) and the electromagnetic force (which repels the positively charged protons).
- Chemical properties: Isotopes of the same element generally have very similar chemical properties. This is because chemical properties are largely determined by the number of electrons, which is the same for isotopes of the same element. However, slight differences may exist due to the isotope's mass affecting reaction rates (kinetic isotope effect).
Applications of Isotopes
The unique properties of isotopes make them invaluable tools in various fields:
1. Radioactive Dating:
Radioactive isotopes, with their known decay rates, are essential for radiometric dating. This technique is used to determine the age of materials such as rocks, fossils, and artifacts. The most famous example is carbon-14 dating, which is used to date organic materials up to around 50,000 years old. Other radioactive isotopes, such as uranium-238 and potassium-40, are used to date much older materials.
2. Medical Applications:
Radioactive isotopes have numerous applications in medicine, including:
- Medical imaging: Isotopes like technetium-99m are used in techniques such as single-photon emission computed tomography (SPECT) and positron emission tomography (PET) scans to visualize internal organs and tissues.
- Radiation therapy: Radioactive isotopes are used to target and destroy cancer cells.
- Radiopharmaceuticals: Radioactive isotopes are incorporated into drugs to target specific tissues or organs, allowing for diagnosis and treatment.
3. Industrial Applications:
Isotopes find uses in various industrial applications, including:
- Tracing: Radioactive isotopes can be used as tracers to track the movement of materials in industrial processes. For example, they can be used to monitor the flow of liquids in pipelines or the wear and tear of machinery.
- Gauging: Isotopes are used in gauges to measure the thickness of materials such as paper, plastics, and metals.
- Sterilization: Gamma radiation from radioactive isotopes is used to sterilize medical equipment and food.
4. Environmental Studies:
Isotopes are used in various environmental studies, including:
- Water tracing: Isotopes like deuterium and oxygen-18 are used to trace the movement of water in the environment.
- Pollution monitoring: Isotopes can be used to track the sources and movement of pollutants in the environment.
- Climate change research: Isotopes are used to study past climates and predict future climate change.
5. Forensic Science:
Isotopic analysis is also employed in forensic science, assisting in various investigations, such as determining the origin of materials or tracing individuals involved in criminal activity.
Isotope Separation
Separating isotopes is a challenging task because they have nearly identical chemical properties. Several methods are employed, each with its own advantages and limitations:
- Gaseous diffusion: This method exploits the slightly different diffusion rates of gases containing different isotopes.
- Gas centrifugation: This method uses centrifugal force to separate isotopes based on their mass differences.
- Laser isotope separation: This method uses lasers to selectively excite and ionize specific isotopes, allowing for their separation.
Isotopic Abundance and Average Atomic Mass
The isotopic abundance refers to the relative proportion of each isotope of an element found in nature. The average atomic mass of an element listed on the periodic table is a weighted average of the masses of its naturally occurring isotopes, taking into account their respective abundances. This average atomic mass is a crucial value used in many chemical calculations.
Conclusion
Isotopes represent a fascinating aspect of atomic structure, demonstrating that atoms of the same element are not necessarily identical in mass. Their diverse properties, stemming from their varying neutron numbers, make them invaluable tools in a wide array of fields, from medicine and industry to environmental science and archaeology. Understanding isotopes is vital for appreciating the complexity and versatility of the atomic world and its impact on our lives. The ongoing research and development in isotope-related technologies promise even more exciting applications in the future. Further study into isotopic ratios and their subtle variations within materials offers invaluable insights across a vast expanse of scientific disciplines. From the depths of geological time scales to the intricacies of biological processes, the study of isotopes continues to reveal profound information about our world. The subtle yet powerful differences between isotopes underscore the rich complexity and surprising diversity within the seemingly simple atom.
Latest Posts
Latest Posts
-
What Is 160 Degrees Fahrenheit In Celsius
Mar 19, 2025
-
Is The Hypotenuse Always The Longest Side
Mar 19, 2025
-
How Many Inches In 12 Yards
Mar 19, 2025
-
What Is The Percent Of 1 50
Mar 19, 2025
-
How Are The Inner Planets Different From The Outer Planets
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Atoms Of The Same Element That Have Different Masses . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
