How Are Positive And Negative Ions Formed
listenit
Mar 19, 2025 · 5 min read
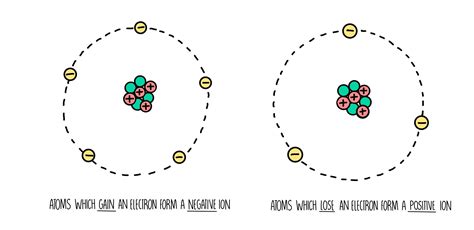
Table of Contents
How Are Positive and Negative Ions Formed?
Understanding the formation of positive and negative ions is fundamental to comprehending a vast range of scientific phenomena, from the behavior of gases in the atmosphere to the intricate workings of chemical reactions and the properties of materials. This comprehensive article delves deep into the mechanisms behind ion formation, exploring the various processes involved and highlighting their significance across different scientific disciplines.
The Basics: What are Ions?
Before diving into the formation processes, let's establish a clear understanding of what ions are. An ion is an atom or molecule that carries a net electrical charge. This charge arises from an imbalance between the number of protons (positively charged particles) and electrons (negatively charged particles) within the atom or molecule.
-
Cations: Ions with a net positive charge are called cations. They are formed when an atom loses one or more electrons. The loss of negatively charged electrons leaves the atom with more protons than electrons, resulting in a positive charge.
-
Anions: Ions with a net negative charge are called anions. They are formed when an atom gains one or more electrons. The addition of negatively charged electrons gives the atom more electrons than protons, resulting in a negative charge.
Mechanisms of Ion Formation: A Detailed Look
The formation of both cations and anions involves several key processes, each driven by the fundamental principles of atomic structure and electrostatics. Let's explore these mechanisms in detail:
1. Ionization by Electron Transfer (Redox Reactions):
This is arguably the most common method of ion formation. It occurs during redox reactions, which involve the transfer of electrons between atoms or molecules. One species loses electrons (oxidation), becoming a cation, while another species gains electrons (reduction), becoming an anion.
Example: Consider the reaction between sodium (Na) and chlorine (Cl). Sodium has a relatively low ionization energy, meaning it readily loses its single valence electron to achieve a stable electron configuration. Chlorine, on the other hand, has a high electron affinity, readily accepting an electron to complete its outer electron shell.
The reaction proceeds as follows:
Na → Na⁺ + e⁻ (Sodium loses an electron, becoming a sodium cation)
Cl + e⁻ → Cl⁻ (Chlorine gains an electron, becoming a chloride anion)
The resulting ions, Na⁺ and Cl⁻, are then electrostatically attracted to each other, forming the ionic compound sodium chloride (NaCl), commonly known as table salt.
2. Ionization by Electron Impact:
This process involves the collision of an atom or molecule with a high-energy electron. The impact can impart enough energy to knock an electron out of the atom or molecule, leaving behind a positively charged ion (cation).
This mechanism is prevalent in various environments, including:
-
Gas Discharge Tubes: In these tubes, a high voltage is applied across a gas, creating a stream of electrons that collide with gas atoms, ionizing them. This process is used in various applications, including lighting and spectroscopy.
-
Mass Spectrometry: Mass spectrometers utilize electron impact ionization to create charged particles that can be separated and analyzed based on their mass-to-charge ratio.
-
The Upper Atmosphere (Ionosphere): High-energy radiation from the sun ionizes atoms and molecules in the Earth's upper atmosphere, creating the ionosphere, a layer of charged particles that plays a crucial role in radio wave propagation.
3. Ionization by Electromagnetic Radiation:
Similar to electron impact ionization, exposure to high-energy electromagnetic radiation (such as X-rays or gamma rays) can also ionize atoms and molecules. The energy of the radiation is absorbed by the atom or molecule, leading to the ejection of an electron and the formation of a cation.
This mechanism is important in:
-
Photoionization: This process is particularly relevant in the upper atmosphere where ultraviolet (UV) radiation from the sun ionizes atmospheric gases.
-
Medical Imaging: X-rays used in medical imaging interact with atoms in the body, causing ionization and generating signals that are detected to create images.
-
Radiation Therapy: High-energy radiation is used to damage cancer cells by causing ionization and disrupting their cellular processes.
4. Ionization by Chemical Reactions:
Beyond simple electron transfer, many chemical reactions lead to the formation of ions. These reactions often involve the interaction of atoms or molecules with strong acids or bases.
Example: The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H₂O). In this reaction, the HCl molecule donates a proton (H⁺) to the NaOH molecule, resulting in the formation of sodium (Na⁺) and chloride (Cl⁻) ions.
HCl + NaOH → NaCl + H₂O
5. Other Ionization Methods:
Other, less common mechanisms for ion formation include:
-
Thermal Ionization: High temperatures can provide enough energy to ionize atoms or molecules.
-
Field Ionization: Strong electric fields can also remove electrons from atoms, leading to ionization.
The Significance of Ion Formation
The formation of ions plays a pivotal role in numerous areas of science and technology:
-
Chemistry: Ions are fundamental to chemical bonding, especially in ionic compounds. Understanding ion formation is crucial for predicting the properties and reactivity of chemical substances.
-
Physics: Ionization is essential in plasma physics, which studies the behavior of ionized gases. Plasmas have various applications, including in fusion energy research and lighting technology.
-
Biology: Ions are essential for many biological processes. For instance, sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and chloride (Cl⁻) ions are crucial for nerve impulse transmission, muscle contraction, and maintaining cellular homeostasis.
-
Materials Science: The properties of many materials are significantly influenced by the presence of ions. Doping semiconductors with ions, for example, alters their electrical conductivity and is crucial in electronics manufacturing.
-
Atmospheric Science: Ion formation in the atmosphere influences weather patterns, air quality, and the formation of clouds.
-
Environmental Science: Monitoring ion concentrations in water and soil helps assess environmental pollution and its impact on ecosystems.
Conclusion:
The formation of positive and negative ions is a fundamental process driven by the interplay of atomic structure and electrostatic forces. Various mechanisms, including electron transfer, electron impact, electromagnetic radiation, and chemical reactions, contribute to the generation of ions. Understanding these processes is critical across multiple scientific disciplines, impacting our understanding of chemical reactions, material properties, biological processes, and environmental phenomena. The continuous exploration and refinement of our knowledge of ion formation will continue to drive advancements in science and technology.
Latest Posts
Latest Posts
-
27 Is What Percent Of 90
Mar 19, 2025
-
What Is Half Of 1 5 Teaspoons
Mar 19, 2025
-
12 Is 40 Of What Number
Mar 19, 2025
-
Where Is Rna Found In Cell
Mar 19, 2025
-
What Is 6 Percent Of 15
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Are Positive And Negative Ions Formed . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
