Is State Of Matter A Physical Change/
listenit
Mar 24, 2025 · 5 min read
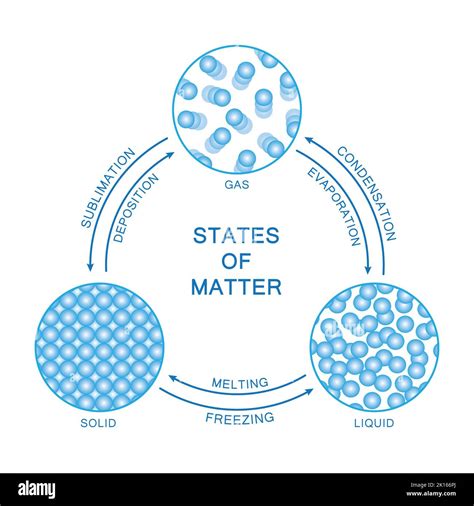
Table of Contents
Is a Change of State of Matter a Physical Change? A Deep Dive
The question of whether a change in the state of matter constitutes a physical change or a chemical change is a fundamental concept in chemistry and physics. While seemingly simple, a thorough understanding requires exploring the definitions of physical and chemical changes, the nature of matter at a molecular level, and the processes involved in transitions between solid, liquid, and gas phases. This article will delve into these aspects, providing a comprehensive answer to the question while also touching upon related concepts like phase diagrams and the role of energy in state changes.
Understanding Physical and Chemical Changes
Before addressing the core question, we need to establish clear definitions:
Physical Change: A physical change alters the form or appearance of a substance but does not change its chemical composition. The molecules themselves remain unchanged. Examples include melting ice, crushing a can, dissolving sugar in water, or bending a piece of metal. These changes are often reversible.
Chemical Change: A chemical change, also known as a chemical reaction, involves a rearrangement of atoms and molecules, resulting in the formation of new substances with different properties. The chemical composition changes. Examples include burning wood, rusting iron, or baking a cake. These changes are typically irreversible.
The States of Matter: Solid, Liquid, and Gas
Matter exists in various states, with the three most common being:
-
Solid: Solids have a definite shape and volume. Their particles (atoms, ions, or molecules) are closely packed and have strong intermolecular forces, resulting in minimal movement. They vibrate in place but don't move freely.
-
Liquid: Liquids have a definite volume but take the shape of their container. Their particles are less tightly packed than in solids and have weaker intermolecular forces, allowing them to move past one another more easily. They exhibit fluidity.
-
Gas: Gases have neither a definite shape nor volume; they expand to fill their container. Their particles are widely dispersed and have very weak intermolecular forces, allowing for extensive movement and random motion. They are highly compressible.
Changes of State: A Physical Transformation
Now, let's directly address the central question: Is a change of state a physical change? The unequivocal answer is yes.
A change of state involves a transition between the solid, liquid, or gaseous phases of matter. These transitions—melting (solid to liquid), freezing (liquid to solid), vaporization (liquid to gas), condensation (gas to liquid), sublimation (solid to gas), and deposition (gas to solid)—are all physical changes.
Why? Because during a change of state, the chemical composition of the substance remains unchanged. The molecules are still the same; only their arrangement and the strength of intermolecular forces are altered.
Melting and Freezing: A Detailed Look
Let's examine melting and freezing as examples to illustrate this point.
When a solid melts, the increased temperature provides enough kinetic energy to overcome the strong intermolecular forces holding the particles in a fixed structure. The particles gain freedom of movement, transitioning into a liquid state. However, the molecules themselves remain identical. Similarly, freezing is simply the reverse process. As temperature decreases, the kinetic energy diminishes, allowing intermolecular forces to dominate, causing the particles to arrange themselves into a more ordered solid structure. The molecular makeup remains the same.
Vaporization and Condensation: Further Examples
Vaporization (including boiling and evaporation) involves overcoming intermolecular forces to transition from a liquid to a gas. The molecules are still the same; they simply possess more kinetic energy and are less constrained by attractive forces. Condensation is the reverse process, where gas molecules lose kinetic energy, leading to increased intermolecular attraction and a return to the liquid phase.
Sublimation and Deposition: Less Common but Equally Physical
Sublimation, the transition from solid directly to gas (e.g., dry ice), and deposition, the transition from gas directly to solid (e.g., frost formation), also represent physical changes. The chemical identity of the substance is not altered. Only the intermolecular forces and the arrangement of particles change.
The Role of Energy in State Changes
Energy plays a crucial role in changes of state. These changes are endothermic (absorbing energy) or exothermic (releasing energy) depending on the direction of the transition.
-
Endothermic Changes: Melting, vaporization, and sublimation are endothermic because they require energy input to overcome the intermolecular forces holding the substance together.
-
Exothermic Changes: Freezing, condensation, and deposition are exothermic because they release energy as the molecules become more ordered and the intermolecular forces strengthen.
This energy exchange is evidence of a physical change, as it affects the kinetic and potential energy of the molecules without altering their chemical composition.
Phase Diagrams: Visualizing State Changes
Phase diagrams graphically represent the conditions of temperature and pressure at which a substance exists in different states. They help visualize the changes of state and the influence of external factors. The lines on a phase diagram represent the equilibrium points between phases. Crossing these lines represents a change of state, again highlighting the physical nature of these transitions.
Distinguishing Physical from Chemical Changes: Key Considerations
While changes of state are unequivocally physical, it's crucial to be able to distinguish physical from chemical changes. Key differences include:
-
Reversibility: Physical changes are often, but not always, reversible. Chemical changes are usually irreversible. While some changes of state may appear difficult to reverse (e.g., shattering glass), the underlying chemical composition remains the same.
-
Energy Changes: Both physical and chemical changes involve energy changes, but the magnitude and type of energy changes can differ significantly.
-
New Substance Formation: Chemical changes result in the formation of new substances with different properties. Physical changes do not create new substances.
Conclusion: Changes of State are Fundamentally Physical
In conclusion, a change of state is definitively a physical change. The chemical identity of the substance remains unaltered; only the physical arrangement of its molecules and the strength of intermolecular forces are affected. Melting, freezing, vaporization, condensation, sublimation, and deposition all represent physical transitions driven by energy changes and governed by the principles of intermolecular forces. Understanding these concepts is crucial for grasping the fundamental nature of matter and the transformations it undergoes. The ability to differentiate between physical and chemical changes is a cornerstone of scientific literacy and allows for better understanding of numerous phenomena in the world around us.
Latest Posts
Latest Posts
-
What Is The Formula For The Compound Magnesium Oxide
Mar 28, 2025
-
What Is The Correct Formula For Calcium Oxide
Mar 28, 2025
-
What Is The Si Base Unit Of Length
Mar 28, 2025
-
What Is The Oxidation State Of Each Element In Coh2
Mar 28, 2025
-
How Many Valence Electrons Are In Boron
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is State Of Matter A Physical Change/ . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
