How Many Valence Electrons Are In Boron
listenit
Mar 28, 2025 · 6 min read
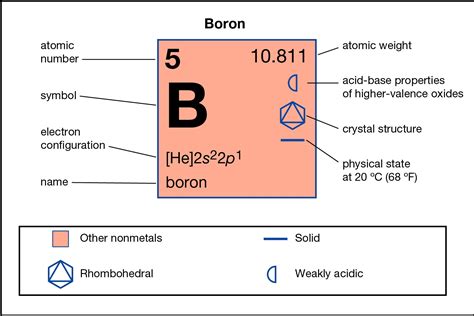
Table of Contents
How Many Valence Electrons Are in Boron? A Deep Dive into Atomic Structure and Bonding
Boron, a metalloid element with the symbol B and atomic number 5, holds a unique position in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is crucial to grasping its chemical behavior and its role in various applications. This article will delve deep into the question: how many valence electrons are in boron? We will explore the concept of valence electrons, boron's electron configuration, its bonding characteristics, and the implications of its valence electron count in different contexts.
Understanding Valence Electrons: The Key to Chemical Behavior
Before we pinpoint the number of valence electrons in boron, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely held and, therefore, play a crucial role in chemical bonding. They determine how an atom will interact with other atoms, influencing its reactivity and the types of compounds it can form. The number of valence electrons directly dictates an element's chemical properties and its position within the periodic table.
Boron's Electron Configuration: Unveiling the Outermost Shell
To determine the number of valence electrons in boron, we need to examine its electron configuration. The atomic number of boron is 5, meaning it has 5 electrons. These electrons are arranged in shells around the nucleus, following specific rules. The electron configuration of boron is 1s²2s²2p¹.
- 1s²: This indicates two electrons in the first electron shell (n=1), which is closest to the nucleus.
- 2s²: This shows two electrons in the second electron shell (n=2), in the s subshell.
- 2p¹: This signifies one electron in the p subshell of the second electron shell (n=2).
The outermost shell for boron is the second shell (n=2). This shell contains a total of three electrons (two from the 2s subshell and one from the 2p subshell). Therefore, boron has three valence electrons.
Boron's Bonding Behavior: A Consequence of Three Valence Electrons
Boron's three valence electrons directly influence its bonding characteristics. It readily forms covalent bonds, sharing its three valence electrons with other atoms to achieve a more stable electron configuration. This is often described as striving to achieve a full octet (eight electrons in the outermost shell), although boron frequently forms compounds with less than eight electrons around it, a phenomenon known as electron deficiency.
Covalent Bonding in Boron Compounds: Examples
Boron's tendency to form covalent bonds is evident in various compounds. Consider the following examples:
-
Boron trifluoride (BF₃): Boron shares its three valence electrons with three fluorine atoms, each of which contributes one electron to form three covalent bonds. This results in a trigonal planar geometry around the boron atom.
-
Diborane (B₂H₆): This molecule is an example of boron's ability to form unusual electron-deficient bonds. It involves three-center two-electron bonds where two boron atoms share a pair of electrons with a hydrogen atom. This is a consequence of boron's attempt to increase its electron count beyond its initial three valence electrons.
-
Boron nitride (BN): Similar to carbon, boron can form extended structures like BN in various forms (such as hexagonal boron nitride), where each boron atom forms covalent bonds with three nitrogen atoms, and each nitrogen atom forms covalent bonds with three boron atoms resulting in a giant covalent structure.
Boron's Role in Various Applications: A Reflection of its Chemical Properties
The unique chemical properties stemming from boron's three valence electrons lead to its use in diverse applications:
-
Semiconductors: Boron is a crucial dopant in semiconductor materials, notably silicon. Its addition introduces p-type conductivity, enhancing the performance of electronic devices. The ability to control and manipulate the conductivity of silicon based on boron doping is a direct outcome of boron's ability to contribute its valence electrons into the silicon crystal lattice.
-
Refractory Materials: Boron compounds, such as boron carbide (B₄C) and boron nitride (BN), are known for their exceptional high-temperature stability and hardness. This makes them invaluable in applications requiring resistance to extreme heat and wear, such as protective coatings and abrasives. The strong covalent bonds in these materials, resulting from boron's sharing of its valence electrons, are responsible for their robust nature.
-
Glass and Ceramics: Boron oxide (B₂O₃) is a significant component in many glasses and ceramics. It improves their properties, including thermal shock resistance and chemical durability. The bonding characteristics of boron contribute to the unique properties of these materials.
-
Medicine and Pharmaceuticals: Boron compounds play a growing role in medicine, particularly in boron neutron capture therapy (BNCT), a type of cancer treatment. Specific boron compounds are designed to accumulate selectively in tumor cells, enabling targeted radiation therapy. The chemical reactivity and bonding capabilities of boron influence the design and effectiveness of such targeted agents.
Beyond the Basics: Isotopes and Beyond
While all boron atoms have three valence electrons, it's important to note the existence of isotopes. Boron exists naturally as a mixture of two stable isotopes, ¹⁰B and ¹¹B, with different abundances. These isotopes have the same number of protons and electrons (and hence, the same number of valence electrons), but differ in the number of neutrons in their nuclei. The isotopic composition doesn't affect the number of valence electrons; only the atomic mass.
Furthermore, understanding boron's three valence electrons is crucial in predicting its various oxidation states. While +3 is the most common, boron can exhibit other oxidation states, though less common, due to factors beyond simple electron counting, which are related to the more complex nature of its bonding and the formation of electron-deficient compounds.
Conclusion: The Significance of Boron's Three Valence Electrons
In conclusion, boron, with its atomic number 5, possesses three valence electrons. This fundamental characteristic dictates its chemical reactivity, bonding behavior, and consequently, its diverse applications in various fields. From its role as a semiconductor dopant to its use in high-temperature materials and medicinal applications, the implications of boron's three valence electrons are far-reaching. Understanding its electron configuration and the resulting bonding properties is essential for appreciating the multifaceted role of this element in science and technology. Further exploration of boron chemistry reveals a rich tapestry of interesting and often unusual chemical behavior arising from its somewhat electron deficient nature despite possessing three valence electrons. The detailed study of boron's interactions continues to reveal new insights into this fascinating element and the fundamental principles governing chemical bonding.
Latest Posts
Latest Posts
-
Least Common Multiple For 18 And 24
Mar 31, 2025
-
What Is 3 4 Equivalent To In Fractions
Mar 31, 2025
-
Where Is Most Freshwater Found On Earth
Mar 31, 2025
-
Simplify The Square Root Of 512
Mar 31, 2025
-
2 1 6 As An Improper Fraction
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Boron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
