Is Salt An Element Compound Or Mixture
listenit
Mar 15, 2025 · 6 min read
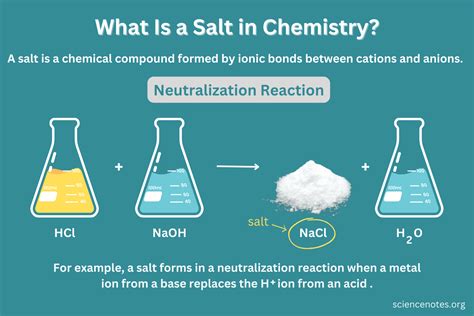
Table of Contents
Is Salt an Element, Compound, or Mixture? A Deep Dive into Sodium Chloride
The seemingly simple question, "Is salt an element, compound, or mixture?" opens a door to a fascinating exploration of chemistry's fundamental building blocks. While the answer might seem straightforward at first glance, a deeper understanding requires examining the definitions of elements, compounds, and mixtures and how they apply to the ubiquitous substance we know as salt. This comprehensive article will delve into the chemical nature of salt, exploring its properties and composition to definitively answer the question and illuminate the broader concepts of matter classification.
Understanding the Basic Building Blocks of Matter
Before we can classify salt, let's clarify the definitions of elements, compounds, and mixtures. These terms are fundamental to understanding the structure and properties of matter.
Elements: The Fundamental Building Blocks
Elements are pure substances that cannot be broken down into simpler substances by chemical means. They are composed of only one type of atom, characterized by a specific number of protons in their nuclei. The periodic table organizes all known elements based on their atomic number and properties. Examples of elements include hydrogen (H), oxygen (O), iron (Fe), and gold (Au). These elements serve as the fundamental building blocks upon which all other substances are constructed.
Compounds: Elements Combined
Compounds are pure substances formed when two or more elements chemically combine in fixed proportions. This chemical combination involves the sharing or transfer of electrons between atoms, forming chemical bonds. The properties of a compound are distinctly different from the properties of its constituent elements. For example, water (H₂O) is a compound formed from the elements hydrogen and oxygen, possessing properties vastly different from either hydrogen gas or oxygen gas. Compounds can only be separated into their constituent elements through chemical processes, not physical ones.
Mixtures: A Blend of Substances
Mixtures are combinations of two or more substances that are not chemically bonded. The substances retain their individual properties within the mixture, and the composition of a mixture can vary. Mixtures can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like sand and water). Mixtures can be separated into their components through physical processes like filtration, distillation, or evaporation.
The Case of Salt: Sodium Chloride (NaCl)
Now, let's focus our attention on salt, commonly known as table salt or sodium chloride (NaCl). Understanding its composition is key to classifying it correctly.
Sodium chloride is formed from the chemical bonding of two elements: sodium (Na) and chlorine (Cl). Sodium is an alkali metal, highly reactive, and soft, while chlorine is a highly toxic, greenish-yellow gas. These two elements, when reacted, undergo a vigorous chemical reaction, exchanging electrons to form an ionic bond. The resulting compound, sodium chloride, is a stable, crystalline solid with entirely different properties from its constituent elements. It's non-reactive, edible (in moderate amounts!), and forms the characteristic cubic crystals we recognize as table salt.
The Ionic Bond in NaCl: A Closer Look
The ionic bond in NaCl is a strong electrostatic attraction between oppositely charged ions. Sodium readily loses one electron to achieve a stable electron configuration, forming a positively charged sodium ion (Na⁺). Chlorine readily gains one electron to achieve a stable configuration, forming a negatively charged chloride ion (Cl⁻). These oppositely charged ions are strongly attracted to each other, forming the crystalline structure of sodium chloride. This strong chemical bond distinguishes sodium chloride from a mere mixture of sodium and chlorine.
Why Salt Isn't a Mixture
The key reason salt is not a mixture lies in the chemical bonding between sodium and chlorine. In a mixture, the individual components retain their chemical identities and properties. You can physically separate sand from water, leaving you with sand and water as they were before mixing. However, you cannot physically separate sodium and chlorine from salt. To obtain sodium and chlorine, you need to perform a chemical process, such as electrolysis, to break the ionic bonds holding the compound together. This demonstrates the fundamental difference between a compound and a mixture.
Salt as a Compound: The Definitive Answer
Given the evidence presented, the definitive answer is clear: salt (sodium chloride) is a compound, not an element or a mixture. It's a pure substance formed by the chemical combination of two elements, sodium and chlorine, in a fixed proportion (1:1). The strong ionic bond between sodium and chloride ions gives it unique properties distinctly different from its constituent elements. This chemical bonding is the critical factor distinguishing compounds from mixtures.
Further Exploration of Salt Properties and Applications
Understanding the chemical nature of salt opens the door to appreciating its diverse applications and properties.
Crystal Structure and Properties
The cubic crystal structure of sodium chloride results from the ordered arrangement of sodium and chloride ions. This regular structure influences its physical properties, including its melting point (801°C), boiling point (1413°C), and solubility in water. The strong ionic bonds require a significant amount of energy to overcome, explaining its relatively high melting and boiling points.
Solubility and Conductivity
Salt's solubility in water is a critical property. When dissolved in water, the ionic bonds break down, and the sodium and chloride ions become surrounded by water molecules. This process leads to an aqueous solution that can conduct electricity, because the freely moving ions can carry an electric charge.
Industrial Applications
Salt has a wide range of industrial applications, including:
- Food preservation: Salt's ability to draw water out of microorganisms inhibits their growth, making it a valuable preservative.
- De-icing roads: Salt lowers the freezing point of water, making it effective in melting ice and snow.
- Chemical manufacturing: Salt serves as a raw material in the production of many chemicals, including sodium hydroxide (lye), chlorine gas, and sodium carbonate (soda ash).
- Water softening: Salt is used in water softeners to regenerate ion-exchange resins, removing minerals like calcium and magnesium that cause water hardness.
Biological Significance
Salt is essential for many biological processes. Sodium and chloride ions play crucial roles in maintaining fluid balance, nerve impulse transmission, and muscle contraction. However, excessive salt intake can be harmful to human health, potentially leading to high blood pressure and other health problems.
Conclusion: The Importance of Understanding Chemical Classification
Classifying substances as elements, compounds, or mixtures is a fundamental concept in chemistry. The seemingly simple question of whether salt is an element, compound, or mixture necessitates a deeper understanding of chemical bonding and the properties of matter. Through examining the chemical composition and properties of sodium chloride, we've definitively established that salt is a compound, demonstrating the importance of precisely defining and understanding the terminology of chemistry. This understanding extends beyond theoretical knowledge, influencing numerous applications, from food preservation to industrial processes and biological functions. The exploration of salt's nature serves as a powerful example of how fundamental chemical concepts underpin many aspects of our daily lives.
Latest Posts
Latest Posts
-
How Many Sigma And Pi Bonds In A Triple Bond
Mar 15, 2025
-
Why Does Active Transport Need Energy
Mar 15, 2025
-
1 Sin 2x 1 Cosx Cosx
Mar 15, 2025
-
The Difference Of 5 And A Number
Mar 15, 2025
-
Least Common Multiple Of 5 4 And 3
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is Salt An Element Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
