Is Gasoline Evaporating A Chemical Change
listenit
Mar 31, 2025 · 5 min read
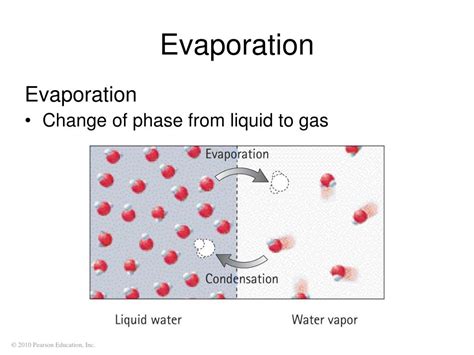
Table of Contents
Is Gasoline Evaporating a Chemical Change? Understanding Physical vs. Chemical Changes
The question of whether gasoline evaporating is a chemical change or a physical change is a common one, especially when exploring the fundamentals of chemistry. The answer, simply put, is no, gasoline evaporating is a physical change. However, understanding why requires a deeper dive into the nature of physical and chemical changes, the composition of gasoline, and the processes involved in evaporation. This article will explore these concepts in detail, providing a comprehensive understanding of this seemingly simple phenomenon.
Understanding Physical and Chemical Changes
Before delving into the specifics of gasoline evaporation, let's establish a clear understanding of the difference between physical and chemical changes. This distinction is crucial for correctly classifying the process.
Physical Changes
A physical change alters the form or appearance of a substance but does not change its chemical composition. Think of it as rearranging the molecules without breaking or forming new bonds. Examples of physical changes include:
- Changes in state: Melting ice (solid to liquid), boiling water (liquid to gas), freezing water (liquid to solid), and deposition (gas to solid).
- Changes in shape: Cutting paper, bending a wire, crushing a can.
- Dissolving: Salt dissolving in water (the salt remains salt, it's just dispersed).
- Mixing: Sand and water mixed together.
Chemical Changes
A chemical change, also known as a chemical reaction, involves the formation of new substances with different chemical properties. This happens when chemical bonds are broken and new bonds are formed, resulting in a fundamental alteration of the molecular structure. Indicators of a chemical change include:
- Formation of a gas: Bubbles forming, fizzing.
- Formation of a precipitate: A solid forming from a solution.
- Color change: A noticeable shift in color.
- Temperature change: Heat is either released (exothermic) or absorbed (endothermic).
- Light emission: Production of light.
The Composition of Gasoline
Gasoline is a complex mixture of hydrocarbons, primarily alkanes, alkenes, and cycloalkanes, with varying chain lengths. These hydrocarbons are organic molecules composed of carbon and hydrogen atoms. The specific composition of gasoline varies depending on the crude oil source and refining processes. However, the key characteristic for our discussion is that the individual hydrocarbon molecules remain intact during evaporation.
The Evaporation Process
Evaporation is a physical process where a liquid transforms into a gas. In the case of gasoline, the heat energy from the surroundings provides the energy needed for the hydrocarbon molecules to overcome their intermolecular forces (forces of attraction between molecules). This allows them to escape from the liquid phase and enter the gaseous phase.
Crucially, the hydrocarbon molecules in the gasoline remain the same chemically. They are not broken down or rearranged into new molecules. They simply transition from a more closely packed liquid state to a more dispersed gaseous state. This is the defining characteristic of a physical change.
Factors Affecting Evaporation Rate
Several factors influence the rate of gasoline evaporation:
- Temperature: Higher temperatures increase the kinetic energy of the molecules, accelerating evaporation.
- Surface area: A larger surface area exposes more molecules to the surrounding air, increasing evaporation.
- Humidity: High humidity reduces the rate of evaporation as the air is already saturated with water vapor.
- Air movement: Wind or air currents remove evaporated gasoline molecules from the vicinity of the liquid surface, promoting further evaporation.
- Volatility of the components: Different hydrocarbons in gasoline have different boiling points. More volatile components evaporate more readily.
Why Gasoline Evaporation Isn't a Chemical Change: A Molecular Perspective
Consider a single molecule of octane (C₈H₁₈), a common component of gasoline. When gasoline evaporates, this octane molecule doesn't break apart into smaller carbon and hydrogen atoms or react with other molecules to form new compounds. Instead, it simply transitions from the liquid phase to the gaseous phase, maintaining its molecular structure. This transition solely involves overcoming intermolecular forces, not breaking or forming intramolecular bonds. This absence of bond breaking or formation is the hallmark of a physical, not a chemical, change.
Common Misconceptions
It's important to address some potential misconceptions surrounding gasoline evaporation:
- Odor: The smell of evaporating gasoline doesn't signify a chemical change. The odor is simply the perception of gasoline molecules in the gaseous phase, interacting with our olfactory sensors.
- Flammability: The fact that gasoline vapor is flammable doesn't mean evaporation is a chemical change. Flammability is a chemical property; however, the combustion of gasoline vapor is a separate chemical reaction (oxidation) that occurs after the evaporation process. Evaporation itself is simply the transition to a gaseous state.
- Potential for oxidation: While prolonged exposure of gasoline to air might lead to slow oxidation (a chemical reaction), this is a separate process and not directly part of the evaporation itself. Evaporation simply presents a larger surface area for oxidation to occur.
Conclusion: A Physical Transformation
In conclusion, gasoline evaporating is unequivocally a physical change. The process involves a transition from the liquid to the gaseous state, with the hydrocarbon molecules retaining their chemical structure. While other chemical reactions might occur alongside or following evaporation (like combustion or slow oxidation), the evaporation process itself is fundamentally a physical transformation involving only a change in state, not a change in chemical composition. Understanding this distinction is crucial for comprehending the nature of matter and the various processes it undergoes. The key takeaway is that changes in state (solid, liquid, gas) are generally physical changes, unless a chemical reaction is inherently part of the phase transition. Gasoline evaporation perfectly illustrates this fundamental principle.
Latest Posts
Latest Posts
-
Is Koh A Base Or Acid
Apr 02, 2025
-
Why Did Small States Object To The Virginia Plan
Apr 02, 2025
-
What Is The Proper Name For Mgf2
Apr 02, 2025
-
Unit Of Measurement For Kinetic Energy
Apr 02, 2025
-
How Do Lichens Contribute To Primary Succession
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Gasoline Evaporating A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
