Is Boiling Water A Chemical Change Or A Physical Change
listenit
Mar 15, 2025 · 5 min read
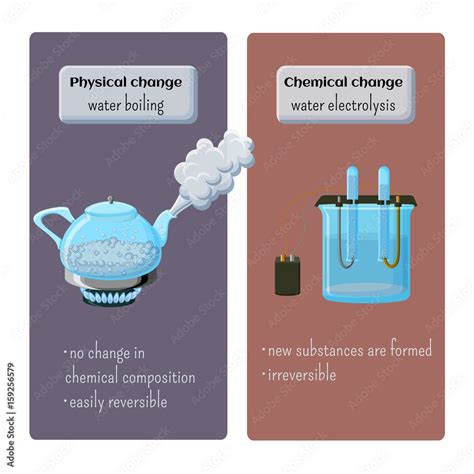
Table of Contents
Is Boiling Water a Chemical Change or a Physical Change?
The question of whether boiling water represents a chemical or physical change is a common one, often arising in science classes and sparking debates among enthusiasts. Understanding the difference between chemical and physical changes is crucial to grasping fundamental concepts in chemistry and physics. This comprehensive article will delve deep into the process of boiling water, examining its characteristics and definitively answering the question. We'll explore the underlying principles, address common misconceptions, and provide practical examples to solidify your understanding.
Understanding Chemical vs. Physical Changes
Before we tackle the boiling water conundrum, let's establish a clear understanding of the difference between chemical and physical changes.
Physical Changes:
A physical change alters the form or appearance of a substance but does not change its chemical composition. Think of it as rearranging the molecules without fundamentally altering them. Examples include:
- Melting ice: Ice (solid water) turns into liquid water, but the H₂O molecules remain the same.
- Cutting paper: The paper changes shape, but it's still paper.
- Dissolving sugar in water: The sugar disappears into the water, but its chemical structure isn't altered. It can be recovered through evaporation.
Key characteristics of physical changes:
- No new substance is formed.
- Changes are usually reversible. (though not always, like breaking a glass)
- Involve changes in physical properties like shape, size, temperature, or state (solid, liquid, gas).
Chemical Changes:
A chemical change, also known as a chemical reaction, involves the formation of one or more new substances with different chemical properties. This often involves the breaking and forming of chemical bonds. Examples include:
- Burning wood: Wood reacts with oxygen to produce ash, smoke, and gases. The original wood is gone, replaced by entirely new substances.
- Rusting iron: Iron reacts with oxygen and water to form iron oxide (rust), a completely different substance.
- Baking a cake: The ingredients undergo chemical reactions, creating a new substance with different properties than the individual ingredients.
Key characteristics of chemical changes:
- New substances are formed with different properties.
- Changes are usually irreversible.
- Often involve energy changes (release or absorption of heat, light, or sound).
Boiling Water: A Deep Dive
Now, let's focus on the central question: Is boiling water a chemical change or a physical change?
The answer is definitively: Boiling water is a physical change.
When water boils, it transitions from its liquid state to its gaseous state (water vapor or steam). However, the chemical composition remains unchanged. The water molecules (H₂O) are still water molecules, simply arranged differently and possessing more kinetic energy. They're farther apart in the gaseous state than in the liquid state.
Evidence Supporting Boiling Water as a Physical Change:
- Reversible Process: If you cool the steam, it condenses back into liquid water, demonstrating the reversibility of the process. This is a hallmark of physical changes.
- No New Substance Formed: The steam produced by boiling water is still H₂O. No new chemical compounds are created. You could collect the steam and condense it back into liquid water, chemically identical to the original water.
- Energy Change is Physical: The energy absorbed during boiling increases the kinetic energy of the water molecules, allowing them to overcome intermolecular forces and transition to a gas. This energy change is associated with a phase transition and not the creation of new chemical bonds.
- Chemical Properties Remain Invariant: The chemical properties of water, such as its reactivity with other substances, remain unchanged after boiling.
Addressing Common Misconceptions:
Some might argue that the formation of steam involves a change in the structure of water molecules, making it a chemical change. However, this is incorrect. While the arrangement of water molecules changes (becoming more dispersed), the molecules themselves remain intact. There's no breaking or forming of chemical bonds between the hydrogen and oxygen atoms within the water molecules.
Similarly, some might point to the potential for dissolved impurities in the water to change during boiling (for example, mineral salts precipitating out). However, these changes relate to the dissolved substances and not the water itself. The water molecule remains fundamentally unchanged.
Expanding on the Concept: Phase Transitions and Physical Changes
Boiling is a specific example of a phase transition, which is a physical change involving a change of state of matter. Other phase transitions include:
- Melting: Solid to liquid
- Freezing: Liquid to solid
- Sublimation: Solid to gas (e.g., dry ice)
- Deposition: Gas to solid (e.g., frost formation)
All these phase transitions are considered physical changes because they only involve changes in the arrangement and energy of molecules, not the fundamental chemical composition of the substance.
Beyond Boiling: Other Examples of Physical Changes
To further solidify our understanding, let's consider other examples of physical changes:
- Crushing a can: The shape of the can changes, but the aluminum remains aluminum.
- Stretching a rubber band: The rubber band changes shape and size, but its chemical makeup stays the same.
- Mixing sand and water: The sand and water are mixed, but neither undergoes a chemical change. They can be separated through physical means.
- Dissolving salt in water: The salt dissolves, forming a solution, but the salt molecules retain their chemical structure. Evaporation would recover the salt.
These examples, along with the boiling of water, clearly demonstrate that physical changes affect the form or appearance of a substance but leave its chemical identity unaltered.
Conclusion: Boiling Water Remains a Physical Change
In conclusion, the process of boiling water is unequivocally a physical change. It involves a change in state from liquid to gas, but the chemical composition of the water (H₂O) remains unchanged. No new substances are formed, and the process is reversible. Understanding this distinction between physical and chemical changes is fundamental to comprehending the basic principles of chemistry and the natural world. While seemingly simple, the concept of boiling water highlights the importance of precise scientific observation and the rigorous application of scientific principles. The subtleties within seemingly straightforward processes underscore the power and beauty of scientific inquiry. Remember to apply this understanding to further explorations of chemistry and physics for a deeper comprehension of the world around us.
Latest Posts
Latest Posts
-
Whats The Square Root Of 28
Mar 15, 2025
-
Where Is The Most Mass Of An Atom Found
Mar 15, 2025
-
What Is The Lowest Common Factor Of 12 And 15
Mar 15, 2025
-
Give The Formula For Plumbous Nitrate
Mar 15, 2025
-
1 And 2 3 As A Decimal
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Water A Chemical Change Or A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
