How To Find The Ph At The Equivalence Point
listenit
Mar 15, 2025 · 6 min read
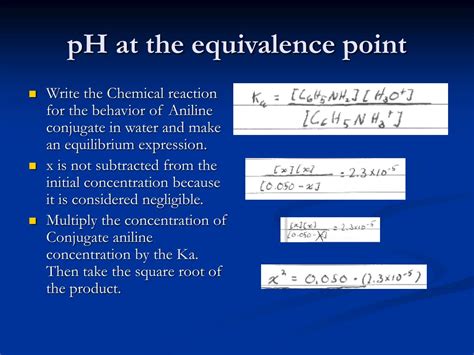
Table of Contents
How to Find the pH at the Equivalence Point: A Comprehensive Guide
Determining the pH at the equivalence point of a titration is crucial for understanding the reaction's stoichiometry and the properties of the resulting solution. This guide will provide a comprehensive understanding of this process, covering various scenarios and techniques.
Understanding the Equivalence Point
The equivalence point in a titration is the point at which the added titrant has completely reacted with the analyte. This means the moles of acid (if titrating a base) or base (if titrating an acid) are stoichiometrically equal to the moles of the substance being titrated. It's a theoretical point, determined by calculations based on the balanced chemical equation. The equivalence point is not the same as the endpoint, which is the point at which the indicator changes color, signifying a visually observable change in the solution. Ideally, the endpoint should be as close as possible to the equivalence point, but slight differences can exist depending on the indicator used.
Methods for Finding the pH at the Equivalence Point
The approach to determining the pH at the equivalence point depends on the nature of the acid and base involved in the titration:
1. Strong Acid - Strong Base Titration
This is the simplest case. Since both the acid and base are completely dissociated, the pH at the equivalence point is 7. This is because the resulting solution is composed of only the conjugate base and conjugate acid of a strong acid and strong base, both of which are very weak and thus do not significantly affect the pH. The autoionization of water then determines the pH, which is 7 at 25°C.
Example: Titration of HCl (strong acid) with NaOH (strong base).
At the equivalence point, the solution contains only NaCl and water. Neither NaCl nor water significantly affects the pH, resulting in a neutral pH of 7.
2. Weak Acid - Strong Base Titration
This scenario is more complex. At the equivalence point, the weak acid has been completely neutralized by the strong base, resulting in a solution containing only the conjugate base of the weak acid. This conjugate base will undergo hydrolysis, reacting with water to produce hydroxide ions (OH⁻), thus making the solution basic.
To find the pH:
-
Determine the concentration of the conjugate base: This requires knowing the initial concentration of the weak acid and the volume of strong base added at the equivalence point. The moles of conjugate base will equal the initial moles of weak acid. Divide the moles of conjugate base by the total volume of the solution to get its concentration.
-
Calculate the Kb of the conjugate base: The Kb (base dissociation constant) is related to the Ka (acid dissociation constant) of the weak acid by the equation: Kw = Ka * Kb, where Kw is the ion product constant of water (1.0 x 10⁻¹⁴ at 25°C).
-
Use an ICE table (Initial, Change, Equilibrium) to calculate the hydroxide ion concentration: Set up an ICE table for the hydrolysis reaction of the conjugate base. Solve for [OH⁻] using the Kb value.
-
Calculate the pOH: pOH = -log[OH⁻]
-
Calculate the pH: pH = 14 - pOH
Example: Titration of acetic acid (weak acid) with NaOH (strong base).
At the equivalence point, the solution contains sodium acetate (the conjugate base). You'd use the steps above to calculate the pH, which will be greater than 7.
3. Weak Base - Strong Acid Titration
Similar to the previous case, but in reverse. At the equivalence point, the weak base has been completely neutralized, leaving only its conjugate acid in solution. This conjugate acid will undergo hydrolysis, producing hydronium ions (H₃O⁺), resulting in an acidic solution.
The process for calculating the pH is analogous to the weak acid - strong base titration:
-
Determine the concentration of the conjugate acid.
-
Calculate the Ka of the conjugate acid: Use the relationship Kw = Ka * Kb, where Kb is the base dissociation constant of the weak base.
-
Use an ICE table to calculate the hydronium ion concentration [H₃O⁺].
-
Calculate the pH: pH = -log[H₃O⁺]
Example: Titration of ammonia (weak base) with HCl (strong acid).
At the equivalence point, the solution contains ammonium chloride (the conjugate acid). The calculations would follow the steps outlined above, yielding a pH less than 7.
4. Polyprotic Acid Titrations
Polyprotic acids have multiple ionizable protons. These titrations have multiple equivalence points, one for each proton. The pH at each equivalence point is calculated differently, depending on the relative strengths of the successive dissociations. The calculations become more complex, often requiring iterative methods or approximation techniques. For example, you might need to consider the individual Ka values and use successive ICE tables for each deprotonation step.
5. Utilizing Titration Curves
A titration curve graphically represents the pH change during a titration. The equivalence point is located where the slope of the curve is steepest. The pH at this point can be determined directly from the curve. Many modern pH meters can automatically record and display titration curves. The steepest part of the curve represents the equivalence point. You can find the corresponding pH from the vertical axis.
Factors Affecting pH at the Equivalence Point
Several factors influence the pH at the equivalence point:
-
Temperature: The ion product constant of water (Kw) is temperature-dependent. Changes in temperature will alter the pH calculations, especially for weak acid-strong base or weak base-strong acid titrations.
-
Ionic strength: High ionic strength can influence the activity coefficients of ions, leading to deviations from ideal behavior and affecting the calculated pH.
-
Concentration of the solutions: The concentration of the acid and base affects the accuracy of the pH calculation, especially for weak acids and bases. Dilute solutions can be more susceptible to errors.
-
Indicator choice: The endpoint of the titration, as indicated by a color change, should be as close to the equivalence point as possible. Incorrect indicator selection can lead to errors in the pH determination at the equivalence point.
Practical Considerations and Error Analysis
-
Accurate measurements: Precise measurements of volumes and concentrations are essential for accurate pH calculations at the equivalence point. Using calibrated glassware and solutions is crucial.
-
Appropriate indicators: Choosing the right indicator that changes color around the pH of the equivalence point is vital for visually determining the endpoint and minimizing error.
-
Temperature control: Maintaining a constant temperature throughout the titration ensures consistent Kw values and minimizes temperature-related errors.
Conclusion
Determining the pH at the equivalence point involves understanding the acid-base chemistry of the reacting species. The process varies depending on the strengths of the acid and base. While strong acid-strong base titrations result in a neutral pH of 7, weak acid-strong base and weak base-strong acid titrations require more complex calculations involving hydrolysis and equilibrium constants. Using titration curves offers a visual approach to determining the equivalence point and its corresponding pH. Careful attention to detail, accurate measurements, and appropriate techniques are key to minimizing errors and obtaining reliable results. This comprehensive guide provides the necessary background and tools to successfully navigate these calculations.
Latest Posts
Latest Posts
-
All Organic Compounds Contain The Element Carbon
Mar 15, 2025
-
12 Is What Percent Of 20
Mar 15, 2025
-
Creatine Phosphate Functions Within The Muscle Cells By
Mar 15, 2025
-
What Is 2 And 2 5 As A Decimal
Mar 15, 2025
-
What Elements In The Same Period Have In Common
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
