All Organic Compounds Contain The Element Carbon
listenit
Mar 15, 2025 · 6 min read
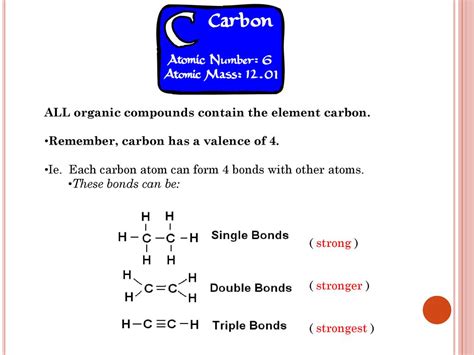
Table of Contents
All Organic Compounds Contain the Element Carbon: A Deep Dive into Organic Chemistry
The statement "all organic compounds contain the element carbon" is a fundamental tenet of organic chemistry. While seemingly simple, this assertion opens the door to a vast and complex world of molecules that form the basis of life and underpin countless applications in our daily lives. This article will delve into the reasons behind this defining characteristic of organic compounds, exploring the unique properties of carbon that allow for its incredible versatility and the exceptions (and why they're not truly exceptions) to this seemingly absolute rule.
The Uniqueness of Carbon: Why Carbon is the Backbone of Life
Carbon's central role in organic chemistry stems from its unique atomic structure and bonding capabilities. Unlike many other elements, carbon possesses four valence electrons, allowing it to form four strong covalent bonds. This tetravalency is the key to carbon's versatility. These bonds can be single, double, or triple bonds, leading to a vast array of possible molecular structures.
Carbon's Ability to Catonate: The Foundation of Complexity
Carbon's ability to form chains, branched structures, and rings (catenation) is unparalleled. This allows for the construction of incredibly large and complex molecules, ranging from simple hydrocarbons to the intricate biopolymers found in living organisms like proteins and DNA. Other elements exhibit catenation to a lesser extent, but none achieve the scale and complexity seen with carbon. Silicon, for example, can form chains, but these are significantly less stable and shorter than carbon chains.
The Diversity of Functional Groups: Determining Molecular Properties
The versatility of carbon isn't limited to its bonding capacity. The presence of different functional groups attached to the carbon backbone dramatically alters the chemical properties of the molecule. A functional group is a specific group of atoms within a molecule that is responsible for characteristic chemical reactions of that molecule. Examples include hydroxyl groups (-OH), carboxyl groups (-COOH), amino groups (-NH2), and carbonyl groups (C=O). The presence and arrangement of these functional groups determine whether a molecule is an alcohol, an acid, an amine, or a ketone, dramatically impacting its reactivity and biological function.
Exploring the Vast Landscape of Organic Compounds
The sheer diversity of organic compounds is staggering. From the simplest hydrocarbon, methane (CH₄), to the complex macromolecules of DNA, the number of possible organic compounds is essentially limitless. This vastness arises from the combinatorial possibilities offered by carbon's ability to form long chains, branched structures, rings, and incorporate various functional groups.
Hydrocarbons: The Building Blocks of Organic Chemistry
Hydrocarbons are organic compounds composed solely of carbon and hydrogen atoms. They form the foundation upon which many other organic molecules are built. Hydrocarbons are further categorized into alkanes (containing only single bonds), alkenes (containing at least one double bond), alkynes (containing at least one triple bond), and aromatic hydrocarbons (containing benzene rings). Their properties vary considerably depending on their structure and chain length. Short-chain hydrocarbons are gases at room temperature, while longer chains are liquids or solids.
Functionalized Hydrocarbons: Expanding the Chemical Repertoire
The addition of functional groups to hydrocarbon backbones significantly expands the chemical diversity of organic compounds. These functional groups introduce new reactive sites, leading to a wider range of chemical properties and biological functions. For instance, the addition of a hydroxyl group (-OH) transforms a hydrocarbon into an alcohol, significantly increasing its polarity and water solubility. Similarly, the addition of a carboxyl group (-COOH) creates a carboxylic acid, a compound with acidic properties.
Biomolecules: The Chemistry of Life
Living organisms rely heavily on carbon-based molecules for their structure and function. Four major classes of biomolecules – carbohydrates, lipids, proteins, and nucleic acids – are all built around carbon backbones. Carbohydrates, composed of carbon, hydrogen, and oxygen, serve as energy sources and structural components. Lipids, including fats and oils, are essential for energy storage, cell membrane structure, and hormone production. Proteins, built from amino acids (containing carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur), perform a vast array of functions, including catalysis, transport, and structural support. Nucleic acids, DNA and RNA, carry genetic information and are crucial for protein synthesis and inheritance.
The (Non-)Exceptions: Addressing Apparent Contradictions
While the statement "all organic compounds contain carbon" is broadly accurate, there are a few compounds traditionally classified as organic that might seem to challenge this rule. However, a closer examination reveals that these apparent exceptions are actually consistent with the underlying principle.
Carbon Dioxide and Carbon Monoxide: The Grey Area
Carbon dioxide (CO₂) and carbon monoxide (CO) are often considered inorganic compounds due to their simple structures and the absence of carbon-carbon bonds. However, they are undeniably carbon-containing compounds and readily participate in reactions that are considered organic processes like photosynthesis and combustion. The classification depends heavily on context and the specific properties under consideration.
Carbides: Compounds with Carbon in an Inorganic Context
Carbides are compounds containing carbon bonded to a metal. While the carbon-metal bonds are significantly different from the carbon-carbon bonds found in most organic compounds, they still involve carbon and are often synthesized using methods common in organic chemistry. Therefore, their classification as inorganic doesn't negate the presence of carbon.
Bicarbonates and Carbonates: Important Carbon-Containing Species
Bicarbonates (HCO₃⁻) and carbonates (CO₃²⁻) are ionic compounds that contain carbon. While found in minerals and other inorganic contexts, they readily participate in biological processes and reactions where they function as carbon sources. Their presence highlights the importance of carbon even within seemingly inorganic contexts.
The Ongoing Relevance of Organic Chemistry
The field of organic chemistry continues to expand rapidly, driven by the constant discovery of new organic compounds and their diverse applications. From the development of new pharmaceuticals and materials to the exploration of sustainable energy sources, organic chemistry plays a critical role in addressing many of the world's most pressing challenges.
Applications in Medicine: The Power of Organic Molecules
Organic chemistry lies at the heart of drug discovery and development. Many pharmaceuticals are organic molecules designed to interact with specific biological targets within the body, such as enzymes or receptors. The precise synthesis and modification of these molecules are crucial for optimizing their efficacy and minimizing side effects.
Materials Science and Nanotechnology: Exploring New Frontiers
Organic chemistry is instrumental in the design and synthesis of new materials with tailored properties. Polymers, composed of long chains of repeating organic units, find extensive use in various applications, ranging from plastics and textiles to advanced composites. Nanotechnology, which involves manipulating materials at the nanoscale, relies heavily on organic chemistry for creating novel nanoscale structures and functional devices.
Sustainable Chemistry and Renewable Energy: Addressing Global Challenges
Organic chemistry is crucial in developing sustainable solutions for environmental and energy challenges. Biofuels derived from organic materials offer a renewable alternative to fossil fuels, while biomass-based polymers provide a more sustainable approach to materials production. Biocatalysis, utilizing enzymes for chemical transformations, offers a greener and more efficient alternative to traditional chemical processes.
Conclusion: The Enduring Significance of Carbon in Organic Chemistry
The statement that all organic compounds contain carbon remains a cornerstone of organic chemistry. Carbon's unique properties – tetravalency, catenation, and the ability to form diverse functional groups – allow for the construction of an incredible variety of molecules, ranging from simple hydrocarbons to the complex biomolecules that underpin life. While some compounds might seem to blur the lines between organic and inorganic, their carbon content ultimately reinforces the central role of carbon in the vast and ever-expanding world of organic chemistry. The continuing advancements in the field promise to unlock even more profound applications and solutions in various areas, further cementing the importance of carbon in the future of science and technology.
Latest Posts
Latest Posts
-
Graph The Inequality Y 2x 1
Mar 15, 2025
-
What Is The Charge Of Iron
Mar 15, 2025
-
What Is A Fraction That Is Equivalent To 3 4
Mar 15, 2025
-
What Are The Factors Of 44
Mar 15, 2025
-
What Is Another Name For The First Law Of Motion
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about All Organic Compounds Contain The Element Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
