What Is The Charge Of Iron
listenit
Mar 15, 2025 · 6 min read
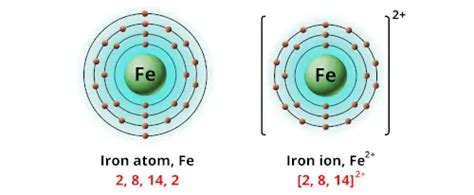
Table of Contents
What is the Charge of Iron? Understanding Oxidation States and Reactivity
Iron, a ubiquitous element crucial to life and industry, doesn't possess a single, fixed charge. Instead, its charge, or more accurately, its oxidation state, varies depending on its chemical environment and bonding partners. This variability is a key factor in iron's diverse reactivity and its importance in biological and industrial processes. This comprehensive article will delve into the complexities of iron's charge, exploring its different oxidation states, the factors influencing them, and the consequences for its chemical behavior.
Understanding Oxidation States
Before we dive into the specific oxidation states of iron, let's establish a clear understanding of what an oxidation state represents. An oxidation state, also known as an oxidation number, is a hypothetical charge assigned to an atom in a molecule or ion, assuming that all bonds are 100% ionic. This means we're assigning electrons to the more electronegative atom in a bond. It's a useful tool for understanding electron transfer and predicting chemical behavior, even though it doesn't reflect the true charge distribution in many covalent compounds.
It's crucial to remember that oxidation states are formal charges; they are not necessarily the actual charges on the atoms. While useful in predicting reactivity, they are a simplified model of a complex reality.
Common Oxidation States of Iron
Iron (Fe) exhibits a variety of oxidation states, but the most common are +2 and +3. These are often denoted as ferrous (Fe²⁺) and ferric (Fe³⁺) respectively. Let's examine each in detail:
Ferrous Iron (Fe²⁺)
Ferrous iron, with an oxidation state of +2, is characterized by its relatively high reactivity. It readily loses another electron to reach the more stable +3 oxidation state. This tendency makes ferrous iron a strong reducing agent, meaning it can donate electrons to other species. Many ferrous compounds are pale green or colorless in solution.
Examples of Ferrous Compounds:
- Iron(II) sulfate (FeSO₄): Used as a dietary supplement and in industrial applications.
- Iron(II) chloride (FeCl₂): Used as a reducing agent and in water treatment.
- Iron(II) oxide (FeO): A black solid found in some minerals.
Ferric Iron (Fe³⁺)
Ferric iron, with an oxidation state of +3, is more stable than ferrous iron. It has a greater tendency to accept electrons rather than donate them, making it a weaker reducing agent compared to Fe²⁺. Many ferric compounds have characteristic yellow-brown or reddish-brown colors in solution.
Examples of Ferric Compounds:
- Iron(III) oxide (Fe₂O₃): The main component of rust and a common pigment (hematite).
- Iron(III) chloride (FeCl₃): Used as a catalyst and in water treatment.
- Iron(III) hydroxide (Fe(OH)₃): A reddish-brown precipitate formed in many reactions involving iron(III).
Less Common Oxidation States of Iron
While +2 and +3 are the most prevalent, iron can exist in other, less common oxidation states under specific conditions:
-
Fe⁰ (zero): This oxidation state represents elemental iron, as found in its metallic form. It's a relatively unreactive element in its pure state but readily reacts with oxygen and water to form oxides and hydroxides.
-
Fe¹⁺ (one): This oxidation state is relatively rare and usually short-lived. It's found in some organometallic compounds and in specific enzyme active sites.
-
Fe⁴⁺ (four): This is a high oxidation state observed in certain complex oxides and in some enzymatic reactions. It's highly oxidizing and usually unstable.
-
Fe⁵⁺ (five): This is an extremely rare and highly oxidizing oxidation state. It's only observed under very specific and unusual conditions.
Factors Influencing Iron's Oxidation State
Several factors determine which oxidation state iron adopts in a particular situation:
-
pH: The acidity or alkalinity of the solution plays a significant role. In acidic conditions, Fe³⁺ is more stable, while in alkaline conditions, Fe²⁺ can be more prevalent. The solubility of iron compounds also changes drastically with pH.
-
Presence of Oxidizing or Reducing Agents: Oxidizing agents, such as oxygen or hydrogen peroxide, tend to favor the formation of Fe³⁺. Conversely, reducing agents, such as sulfides or ascorbic acid, promote the formation of Fe²⁺.
-
Ligands: Ligands are molecules or ions that bond to the central iron ion. Different ligands can stabilize different oxidation states of iron, influencing the equilibrium between Fe²⁺ and Fe³⁺. For example, some ligands strongly stabilize Fe²⁺, while others favor Fe³⁺. This phenomenon is crucial in biological systems where iron is bound to proteins with specific ligands.
-
Temperature: Higher temperatures can sometimes shift the equilibrium between different oxidation states.
-
Pressure: Pressure can also subtly affect the equilibrium between different oxidation states, although the impact is generally less significant than other factors.
The Importance of Iron's Variable Charge
The ability of iron to exist in multiple oxidation states is essential for its numerous applications and its role in biological systems:
Biological Significance
-
Hemoglobin and Myoglobin: Iron in the +2 oxidation state is crucial in hemoglobin and myoglobin, the proteins responsible for oxygen transport and storage in the body. The reversible binding and release of oxygen depend on the iron's ability to switch between different oxidation states.
-
Cytochromes: These iron-containing proteins are involved in electron transport chains in respiration and photosynthesis. The ability of iron to readily accept and donate electrons in different oxidation states is critical for these processes.
-
Enzymes: Many enzymes rely on iron as a cofactor, participating in various catalytic reactions where iron cycles between different oxidation states.
Industrial Applications
-
Steel Production: Iron's diverse oxidation states influence its properties in steel production. Controlling the oxidation state of iron during the manufacturing process allows for the precise tailoring of steel's properties, such as strength, hardness, and ductility.
-
Catalysis: Iron compounds are used as catalysts in numerous industrial processes, exploiting their ability to switch oxidation states and facilitate chemical reactions. Examples include the Haber-Bosch process for ammonia synthesis and various oxidation and reduction reactions.
-
Pigments: Iron oxides in different oxidation states are widely used as pigments in paints, coatings, and cosmetics, providing a wide range of colors from yellow to red and brown.
Analytical Techniques for Determining Iron's Oxidation State
Determining the oxidation state of iron in a sample requires specific analytical techniques. Several methods are available:
-
Titration: Redox titrations can be employed to determine the amount of Fe²⁺ or Fe³⁺ in a sample. This involves reacting the sample with a standardized solution of an oxidizing or reducing agent and monitoring the change in solution potential.
-
Spectroscopy: Spectroscopic techniques, such as UV-Vis spectroscopy and Mössbauer spectroscopy, can provide information about the electronic structure of iron and thereby infer its oxidation state. Each oxidation state exhibits unique spectral features.
-
Electrochemical Methods: Techniques like cyclic voltammetry can be used to study the redox properties of iron and determine the potential for different oxidation states.
-
X-ray Absorption Spectroscopy (XAS): This powerful technique provides detailed information about the local environment around iron atoms, including their oxidation state and coordination geometry.
Conclusion
The charge of iron is not a fixed quantity; rather, it's a variable that reflects its diverse reactivity and crucial roles in both biological and industrial settings. Understanding the various oxidation states of iron, the factors that influence them, and the analytical techniques used to determine them is essential for researchers, engineers, and anyone interested in the fascinating chemistry of this ubiquitous element. The complexity of iron's redox chemistry underscores its multifaceted importance and continued relevance in scientific research and technological advancement. Further research continues to unravel the nuances of iron's redox behavior, offering potential applications in areas like new materials science, medicine, and sustainable energy.
Latest Posts
Latest Posts
-
What Is 170 Degrees C In Fahrenheit
Mar 17, 2025
-
175 Of What Number Is 42
Mar 17, 2025
-
What Percent Is 20 Out Of 50
Mar 17, 2025
-
What Is The Systematic Name For Mg No3 2
Mar 17, 2025
-
What Is 5 12 As A Decimal
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of Iron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
