How To Find The Charge Of Transition Metals
listenit
Mar 28, 2025 · 5 min read
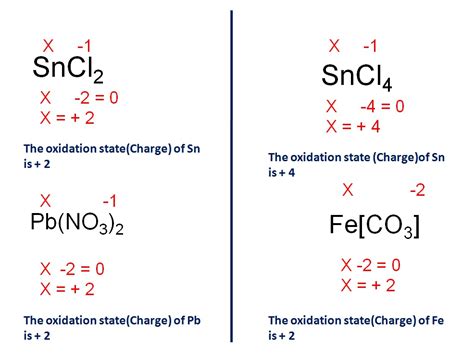
Table of Contents
How to Find the Charge of Transition Metals: A Comprehensive Guide
Transition metals, residing in the d-block of the periodic table, are renowned for their variable oxidation states, making determining their charge a fascinating and often challenging task. Unlike alkali metals with predictable +1 charges or alkaline earth metals with +2, transition metals can exhibit multiple charges, complicating the process. This comprehensive guide will equip you with the tools and understanding necessary to confidently determine the charge of transition metals in various compounds.
Understanding Transition Metal Chemistry: The Key to Finding Charges
The key to understanding transition metal charges lies in their electronic configuration. Transition metals possess partially filled d-orbitals, allowing them to lose varying numbers of electrons to achieve stability. This variability is the root of their diverse oxidation states and complex chemistry. Factors influencing the charge include:
1. Ligand Field Stabilization Energy (LFSE):
Ligands, the atoms or molecules surrounding the central transition metal ion, significantly influence its charge. The interaction between the metal ion's d-orbitals and the ligand orbitals leads to LFSE. Certain ligand arrangements and electron configurations minimize the energy of the complex, favoring specific charges.
2. Oxidation State:
The oxidation state (or oxidation number) represents the charge an atom would have if all bonds were completely ionic. This is a crucial piece of information when determining the charge of a transition metal. It's essential to remember that oxidation states are formal charges and don't necessarily reflect the true charge distribution within the molecule.
3. Electronegativity:
The electronegativity difference between the transition metal and the ligands impacts charge distribution. Highly electronegative ligands tend to pull electron density away from the metal, potentially leading to higher positive charges on the metal center.
4. Coordination Number:
The number of ligands directly bonded to the central metal ion, known as the coordination number, influences the geometry and electronic arrangement around the metal, and consequently, its charge. Different coordination numbers often correlate with different preferred oxidation states.
Methods for Determining the Charge of Transition Metals
Several methods can be employed to determine the charge of transition metal ions in compounds:
1. Using the Charge Balance Method:
This is perhaps the most straightforward method. It relies on the principle of charge neutrality in compounds. The sum of the charges of all atoms in a neutral compound must equal zero. This method is particularly effective for simple ionic compounds.
Example: Consider the compound FeCl<sub>3</sub>. Chlorine (Cl) typically has a charge of -1. Since there are three chlorine atoms, the total negative charge is -3. To maintain charge neutrality, the iron (Fe) atom must have a charge of +3. Therefore, the charge of iron in FeCl<sub>3</sub> is +3.
2. Analyzing Oxidation States:
Determining the oxidation state of the transition metal is a crucial step in identifying its charge. For simple compounds, assigning known oxidation states to other atoms allows the calculation of the transition metal's oxidation state.
Example: In KMnO<sub>4</sub> (potassium permanganate), potassium (K) has a +1 charge and oxygen (O) has a -2 charge. Let x be the oxidation state of manganese (Mn). Therefore, (+1) + x + 4(-2) = 0. Solving for x, we get x = +7. Hence, the oxidation state of manganese in KMnO<sub>4</sub> is +7. While not precisely the charge, the oxidation state strongly suggests the Mn ion carries a significant positive charge.
3. Utilizing Spectroscopic Techniques:
Advanced techniques like X-ray photoelectron spectroscopy (XPS) and UV-Vis spectroscopy can provide direct or indirect information about the charge distribution and oxidation state of transition metals within a compound. XPS measures core-level binding energies, which are sensitive to the charge state of the atom. UV-Vis spectroscopy examines the electronic transitions within the complex, offering insights into the electronic configuration and thus, the charge of the metal center. However, these methods require specialized equipment and expertise.
4. Employing Magnetic Properties:
The magnetic properties of transition metal complexes are intimately linked to their electronic configuration and, hence, their charge. Paramagnetic complexes possess unpaired electrons, leading to attraction towards a magnetic field, while diamagnetic complexes have all electrons paired and are repelled by magnetic fields. Analyzing the magnetic susceptibility of a compound can provide information about the number of unpaired electrons and, indirectly, the charge of the transition metal.
5. Considering the Ligand's Effect:
The nature of the ligands surrounding the transition metal plays a crucial role in determining its charge. Strong-field ligands cause a larger splitting of the d-orbitals, favoring higher oxidation states. Weak-field ligands, conversely, result in smaller splitting and often lead to lower oxidation states. This consideration is particularly important when dealing with coordination complexes.
Common Oxidation States of Transition Metals: A Quick Reference
While transition metals exhibit variable oxidation states, some are more common than others. Familiarizing yourself with these common states can aid in predicting the charge:
- Iron (Fe): +2 (ferrous), +3 (ferric)
- Copper (Cu): +1 (cuprous), +2 (cupric)
- Chromium (Cr): +2, +3, +6
- Manganese (Mn): +2, +4, +7
- Cobalt (Co): +2, +3
- Nickel (Ni): +2
- Zinc (Zn): +2
Tackling Complex Cases: Advanced Techniques
Determining the charge in complex compounds can be more challenging. These strategies are helpful in such situations:
-
Stepwise Charge Assignment: In complex compounds, systematically assign charges to known components first, working towards the unknown transition metal charge.
-
Structural Considerations: The molecular structure can provide clues about the coordination environment and thus the likely charge.
-
Comparative Analysis: Comparing the properties of the unknown compound to those of similar compounds with known charges can aid in deduction.
Conclusion: Mastering the Art of Determining Transition Metal Charges
Determining the charge of transition metals requires a multi-faceted approach, combining knowledge of their electronic structure, oxidation states, ligand effects, and potentially spectroscopic or magnetic data. While straightforward methods exist for simpler compounds, complex molecules may require a more nuanced and comprehensive analysis. By understanding the principles outlined in this guide and systematically applying the described methods, you'll be well-equipped to confidently tackle the challenges of transition metal charge determination. Remember that practice is key – the more you work with these concepts and examples, the more intuitive it will become. Further exploration into advanced techniques such as computational chemistry can provide even deeper insight into this fascinating area of chemistry.
Latest Posts
Latest Posts
-
What Is 8 12 As A Percent
Mar 31, 2025
-
How Many Electrons Do Chlorine Have
Mar 31, 2025
-
Draw A Lewis Structure For Co2
Mar 31, 2025
-
How Many Atoms In Sulfuric Acid
Mar 31, 2025
-
What Is The Decimal For 4 25
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Charge Of Transition Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
