How Many Electrons Do Chlorine Have
listenit
Mar 31, 2025 · 7 min read
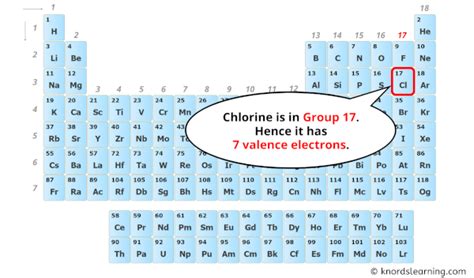
Table of Contents
How Many Electrons Does Chlorine Have? A Deep Dive into Atomic Structure
Chlorine, a vital element in various aspects of our lives, from table salt to water purification, is fascinating from a chemical perspective. One of the fundamental questions about any element is: how many electrons does it possess? This seemingly simple question opens the door to understanding chlorine's chemical behavior, its place in the periodic table, and its role in countless chemical reactions. This comprehensive article will explore the answer to this question and delve into the broader implications of chlorine's electron configuration.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before diving into the specifics of chlorine, let's establish a fundamental understanding of atomic structure. Atoms, the building blocks of matter, are composed of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's atomic number and determines its identity.
- Neutrons: Neutrally charged particles also found in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in distinct energy levels or shells. These electrons are responsible for the chemical properties of an element and its interactions with other atoms.
The number of protons and electrons in a neutral atom are always equal, resulting in a net neutral charge. However, atoms can gain or lose electrons to form ions, resulting in a net positive (cation) or negative (anion) charge.
Chlorine's Atomic Number and Electron Configuration
Chlorine's atomic number is 17. This means that a neutral chlorine atom has 17 protons in its nucleus. Crucially, this also means it possesses 17 electrons orbiting the nucleus. This electron configuration is what dictates chlorine's chemical reactivity and its position in the periodic table.
The electrons are arranged in specific energy levels or shells around the nucleus according to the Aufbau principle and Hund's rule. For chlorine, the electron configuration is as follows:
1s² 2s² 2p⁶ 3s² 3p⁵
Let's break this down:
- 1s²: The first energy level (n=1) contains the s subshell, which can hold up to two electrons. Chlorine has two electrons in this lowest energy level.
- 2s²: The second energy level (n=2) also contains an s subshell with two electrons.
- 2p⁶: The second energy level also has a p subshell, which can accommodate up to six electrons. Chlorine fills this subshell completely.
- 3s²: The third energy level (n=3) starts with an s subshell holding two electrons.
- 3p⁵: The third energy level's p subshell contains five electrons. This is crucial to understanding chlorine's reactivity. A complete p subshell holds six electrons, so chlorine is only one electron short of a stable octet.
Chlorine's Reactivity and the Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons (or two for the first shell). This stable configuration minimizes their energy and increases their stability.
Because chlorine has seven electrons in its outermost shell (the 3rd energy level), it readily gains one electron to achieve a stable octet. This results in the formation of a chloride ion (Cl⁻), which has a negative charge due to the extra electron. This high electronegativity is a defining characteristic of chlorine and explains its strong tendency to form ionic bonds with other elements, especially metals.
Isotopes of Chlorine and Electron Count
While the number of protons defines an element, the number of neutrons can vary. These variations are called isotopes. Chlorine has two naturally occurring isotopes:
- Chlorine-35 (³⁵Cl): This isotope comprises approximately 76% of naturally occurring chlorine. It has 17 protons and 18 neutrons. Despite the difference in neutron number, it still has 17 electrons in a neutral atom.
- Chlorine-37 (³⁷Cl): This isotope makes up about 24% of naturally occurring chlorine. It has 17 protons and 20 neutrons. Again, a neutral atom of this isotope has 17 electrons.
Regardless of the isotope, the number of electrons in a neutral chlorine atom remains constant at 17. The difference in neutron number affects the mass of the atom but not its chemical properties or electron count.
Chlorine's Role in Chemistry and Everyday Life
Chlorine's 17 electrons and its resulting chemical reactivity are responsible for its wide-ranging applications:
1. Salt Production:
The most common compound of chlorine is sodium chloride (NaCl), commonly known as table salt. Chlorine readily reacts with sodium (a highly reactive metal) to form this ionic compound, where chlorine gains an electron and sodium loses one.
2. Water Purification:
Chlorine is a powerful disinfectant used to purify drinking water and swimming pools. It kills harmful bacteria and viruses by reacting with their cellular components, preventing waterborne diseases.
3. Industrial Applications:
Chlorine is used extensively in the production of various chemicals, including plastics (PVC), solvents, and pesticides. Its reactivity makes it a versatile building block for many industrial processes.
4. Medical Applications:
Certain chlorine compounds have medical applications, though this area requires careful consideration due to chlorine's potential toxicity. Some chlorine-containing compounds are used as disinfectants and antiseptics.
Understanding Chlorine's Electron Configuration: A Key to its Properties
The seemingly simple answer – 17 electrons – unlocks a deeper understanding of chlorine's behavior. Its electron configuration, with seven valence electrons, explains its high reactivity, its tendency to form negative ions (Cl⁻), and its crucial role in numerous chemical reactions and everyday applications. Knowing the number of electrons isn't just about memorization; it's the key to comprehending the fascinating world of chemical bonding, reactivity, and the significance of this ubiquitous element.
Beyond the Basics: Exploring Advanced Concepts
The discussion above provides a foundational understanding of chlorine's electron count. However, several advanced concepts build upon this foundation:
1. Electron Shells and Subshells: A Deeper Dive
The simple representation of electron configuration (1s² 2s² 2p⁶ 3s² 3p⁵) masks the complexity of electron behavior. Electrons within subshells (s, p, d, f) occupy orbitals, which are regions of space where the probability of finding an electron is high. These orbitals have specific shapes and orientations, and understanding these nuances is crucial in advanced chemistry.
2. Quantum Numbers and Electron Spin
Each electron in a chlorine atom can be described by a set of four quantum numbers: principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). These numbers provide a comprehensive description of the electron's energy, shape of its orbital, orientation in space, and spin.
3. Ionization Energy and Electron Affinity
Chlorine's high electron affinity – its tendency to gain an electron – reflects its electron configuration. Similarly, its ionization energy (the energy needed to remove an electron) is relatively high because removing an electron disrupts the stable octet arrangement.
4. Molecular Orbital Theory
When chlorine forms bonds with other atoms, its atomic orbitals combine to form molecular orbitals. This theory provides a more sophisticated understanding of bonding in molecules containing chlorine.
5. Spectroscopy and Electron Transitions
Spectroscopic techniques, such as UV-Vis spectroscopy, can probe electron transitions within chlorine atoms and molecules. By analyzing the absorption or emission of light, we can gain insights into the energy levels and electronic structure of chlorine.
Conclusion: The Significance of Understanding Electron Count
The seemingly straightforward question, "How many electrons does chlorine have?" leads us on a journey into the fascinating world of atomic structure and chemical behavior. Understanding that a neutral chlorine atom possesses 17 electrons is fundamental to grasping its reactivity, its role in forming compounds, and its diverse applications in various fields. This knowledge forms the basis for understanding more complex concepts in chemistry, providing a crucial foundation for further exploration of this essential element. The 17 electrons aren't just a number; they are the key to unlocking chlorine's secrets.
Latest Posts
Latest Posts
-
What Is All The Colors Combined
Apr 01, 2025
-
How Does Friction Force Affect Motion
Apr 01, 2025
-
The Enzyme That Unwinds Dna Is
Apr 01, 2025
-
Write A Balanced Overall Reaction From These Unbalanced Half Reactions
Apr 01, 2025
-
What Is The Reciprocal Of 1 25
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Do Chlorine Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
