Draw A Lewis Structure For Co2
listenit
Mar 31, 2025 · 5 min read
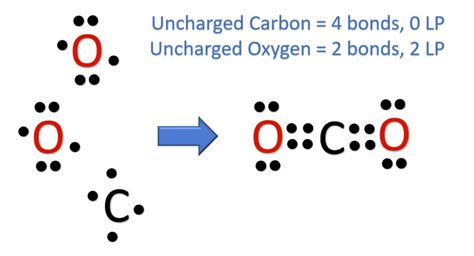
Table of Contents
Drawing the Lewis Structure for CO2: A Comprehensive Guide
Carbon dioxide (CO2) is a ubiquitous molecule found in the atmosphere, playing a crucial role in various natural processes and human activities. Understanding its structure is fundamental to comprehending its properties and behavior. This article provides a step-by-step guide on how to draw the Lewis structure for CO2, explaining the underlying principles and reasoning behind each step. We will delve into the concept of valence electrons, formal charges, resonance structures, and the implications of the final structure on CO2's properties.
Understanding Lewis Structures
A Lewis structure, also known as a Lewis dot diagram, is a simplified representation of a molecule's valence electrons and bonding. It visually depicts the arrangement of atoms and the shared electrons that form covalent bonds. These structures are crucial for predicting molecular geometry, polarity, and reactivity. The central goal is to satisfy the octet rule (or duet rule for hydrogen) for all atoms involved, meaning each atom aims to have eight electrons in its valence shell. Exceptions exist, but understanding the octet rule is a cornerstone of Lewis structure construction.
Step-by-Step Guide to Drawing the Lewis Structure of CO2
Let's break down the process of drawing the Lewis structure for CO2:
Step 1: Count Valence Electrons
The first and most crucial step is determining the total number of valence electrons available. Carbon (C) is in group 14 and has four valence electrons. Oxygen (O) is in group 16 and has six valence electrons each. Since there are two oxygen atoms, the total number of valence electrons in CO2 is:
4 (C) + 2 * 6 (O) = 16 valence electrons
Step 2: Identify the Central Atom
The central atom is typically the least electronegative atom, which is carbon in this case. Oxygen is more electronegative than carbon. Therefore, carbon is placed in the center, with the two oxygen atoms bonded to it.
Step 3: Form Single Bonds
Connect the central carbon atom to each oxygen atom with a single bond. Each single bond consists of two electrons, so we've used four electrons (2 bonds * 2 electrons/bond = 4 electrons).
Step 4: Distribute Remaining Electrons
We started with 16 valence electrons and have used 4, leaving 12 electrons to distribute. We start by completing the octets of the outer atoms (oxygen) first. Each oxygen atom needs six more electrons to complete its octet (8 electrons - 2 electrons from the single bond = 6 electrons). We distribute the remaining 12 electrons (6 electrons per oxygen atom) as lone pairs around the oxygen atoms.
At this point, our structure looks like this: O-C-O, with three lone pairs around each oxygen. However, Carbon only has 4 electrons around it, not satisfying the octet rule.
Step 5: Satisfying the Octet Rule – Double Bonds
To satisfy the octet rule for carbon, we need to form double bonds between the carbon atom and each oxygen atom. This involves moving two lone pairs from each oxygen atom to form a double bond with the carbon. Each double bond consists of four electrons (two electron pairs).
Now, the carbon atom has eight electrons around it (four from two double bonds), and each oxygen atom still has eight electrons around it (two from the double bond + six from three lone pairs). This satisfies the octet rule for all atoms.
Step 6: Formal Charges
Calculating formal charges helps to determine the most stable Lewis structure. The formal charge is the difference between the number of valence electrons in an isolated atom and the number of electrons assigned to that atom in the Lewis structure. The formula for formal charge is:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (1/2 * Bonding Electrons)
Let's calculate the formal charges for our CO2 structure:
- Carbon: 4 (valence) - 0 (non-bonding) - (1/2 * 8 (bonding)) = 0
- Oxygen (each): 6 (valence) - 4 (non-bonding) - (1/2 * 4 (bonding)) = 0
Since all formal charges are zero, this Lewis structure is the most stable representation of CO2.
Step 7: Resonance Structures
For CO2, we can draw two equivalent resonance structures. The electrons in the double bonds can be delocalized, meaning they are not strictly localized between one carbon-oxygen pair but are shared across both carbon-oxygen bonds. This leads to the concept of resonance hybrids where the actual structure is a blend of both resonance structures.
Resonance Structure 1: O=C-O
Resonance Structure 2: O-C=O
Both structures are equally valid and contribute to the overall structure of CO2. The actual molecule is a hybrid of these two structures, with the electrons delocalized across both C=O bonds.
Implications of the Lewis Structure of CO2
The Lewis structure reveals several key properties of CO2:
-
Linear Geometry: The linear arrangement of atoms (O=C=O) results in a linear molecular geometry. This affects properties such as the dipole moment.
-
Nonpolar Molecule: Despite the polar C=O bonds, the linear geometry causes the bond dipoles to cancel each other out, resulting in a nonpolar molecule. This has implications for its solubility and interactions with other molecules.
-
Strong C=O Bonds: The double bonds between carbon and oxygen are relatively strong, contributing to the stability of the molecule.
-
Greenhouse Gas: The structure and bonding in CO2 allow it to absorb and emit infrared radiation, making it a potent greenhouse gas.
Advanced Concepts and Exceptions to the Octet Rule
While the octet rule provides a useful framework, there are exceptions. Some molecules may have atoms with fewer or more than eight electrons in their valence shell. In the case of CO2, the molecule adheres to the octet rule perfectly. However, understanding exceptions is crucial for more advanced Lewis structure applications. For example, molecules with expanded octets (more than eight electrons around a central atom) are common in molecules containing elements from period 3 and beyond.
Conclusion
Drawing the Lewis structure for CO2 is a fundamental exercise in understanding chemical bonding and molecular structure. By systematically following the steps outlined above, one can confidently represent the molecule's electron arrangement and predict some of its important properties. The understanding of valence electrons, formal charges, and resonance structures is key to successfully constructing the Lewis structure and interpreting its implications for the physical and chemical behavior of the molecule. Remember that practice is essential for mastering this skill. Through repetitive exercises and a thorough understanding of the underlying principles, you can effectively utilize Lewis structures to predict and explain the characteristics of numerous molecules.
Latest Posts
Latest Posts
-
The Enzyme That Unwinds Dna Is
Apr 01, 2025
-
Write A Balanced Overall Reaction From These Unbalanced Half Reactions
Apr 01, 2025
-
What Is The Reciprocal Of 1 25
Apr 01, 2025
-
Hcl Ca Oh 2 Balanced Equation
Apr 01, 2025
-
Is 6 7 A Rational Number
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Draw A Lewis Structure For Co2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
