Hcl Ca Oh 2 Balanced Equation
listenit
Apr 01, 2025 · 5 min read
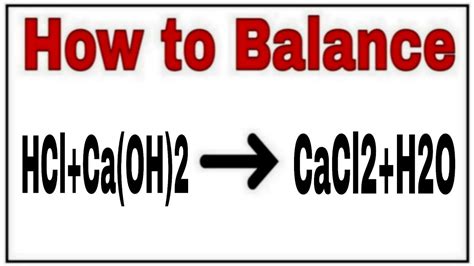
Table of Contents
Understanding the Balanced Equation for the Reaction of HCL and Ca(OH)₂
The reaction between hydrochloric acid (HCl) and calcium hydroxide (Ca(OH)₂) is a classic example of an acid-base neutralization reaction. Understanding this reaction, including its balanced chemical equation, is fundamental to various chemistry concepts, from stoichiometry to titration. This comprehensive guide will delve into the details of this reaction, exploring its balanced equation, the underlying principles, and practical applications.
The Balanced Chemical Equation
The reaction between HCl and Ca(OH)₂ produces calcium chloride (CaCl₂) and water (H₂O). The unbalanced equation looks like this:
HCl + Ca(OH)₂ → CaCl₂ + H₂O
This equation is unbalanced because the number of atoms of each element isn't equal on both sides. To balance it, we need to adjust the coefficients (the numbers in front of the chemical formulas) to ensure mass conservation. The balanced equation is:
2HCl + Ca(OH)₂ → CaCl₂ + 2H₂O
This balanced equation shows that two molecules of hydrochloric acid react with one molecule of calcium hydroxide to produce one molecule of calcium chloride and two molecules of water. Notice how the number of hydrogen (H), chlorine (Cl), calcium (Ca), and oxygen (O) atoms are equal on both the reactant and product sides.
Understanding the Balancing Process
Balancing chemical equations is crucial because it reflects the Law of Conservation of Mass, which states that matter cannot be created or destroyed in a chemical reaction. The total mass of the reactants must equal the total mass of the products. Therefore, the number of atoms of each element must be the same on both sides of the equation.
The balancing process often involves a trial-and-error approach. However, a systematic approach can simplify the process:
-
Start with the most complex molecule: In this case, Ca(OH)₂ is the most complex. We have one calcium atom, two oxygen atoms, and two hydrogen atoms on the reactant side.
-
Balance the metal atoms: There's one calcium atom on the reactant side (in Ca(OH)₂), so we need one calcium atom on the product side. This is already the case in CaCl₂.
-
Balance the polyatomic ions (if any): In this reaction, the hydroxide ion (OH)⁻ is a polyatomic ion. We have two hydroxide ions on the reactant side, which means we have two oxygen atoms and two hydrogen atoms. To balance this, we need two water molecules on the product side.
-
Balance the remaining atoms: Now, let's look at the chlorine atoms. We have two chlorine atoms on the product side (in CaCl₂). Therefore, we need two HCl molecules on the reactant side to balance the chlorine atoms.
-
Verify the balance: After adjusting the coefficients, double-check if the number of atoms of each element is the same on both sides of the equation.
Types of Reactions Involved
The reaction between HCl and Ca(OH)₂ is primarily a neutralization reaction. Neutralization reactions involve the reaction between an acid and a base to form a salt and water. In this specific case:
- HCl is a strong acid, meaning it completely dissociates into its ions (H⁺ and Cl⁻) in water.
- Ca(OH)₂ is a strong base, meaning it also completely dissociates into its ions (Ca²⁺ and 2OH⁻) in water.
The reaction can also be considered a double displacement reaction or metathesis reaction. In these types of reactions, the cations and anions of two different compounds exchange places to form two new compounds.
Ionic Equation and Net Ionic Equation
To gain a deeper understanding of the reaction at the ionic level, we can write the ionic equation and the net ionic equation.
Ionic Equation: This equation shows all the ions involved in the reaction. Since HCl and Ca(OH)₂ are strong electrolytes, they dissociate completely in aqueous solutions.
2H⁺(aq) + 2Cl⁻(aq) + Ca²⁺(aq) + 2OH⁻(aq) → Ca²⁺(aq) + 2Cl⁻(aq) + 2H₂O(l)
Net Ionic Equation: This equation shows only the ions that participate in the reaction. The spectator ions (ions that appear on both sides of the ionic equation and do not participate in the reaction) are removed. In this case, Ca²⁺ and Cl⁻ are spectator ions.
2H⁺(aq) + 2OH⁻(aq) → 2H₂O(l)
This simplified equation highlights the essence of the neutralization reaction: the combination of hydrogen ions (H⁺) and hydroxide ions (OH⁻) to form water.
Applications and Importance
The reaction between HCl and Ca(OH)₂ has several practical applications:
-
Titration: This reaction is commonly used in titrations to determine the concentration of an unknown acid or base. By carefully measuring the volume of HCl required to neutralize a known volume of Ca(OH)₂, the concentration of Ca(OH)₂ can be calculated. This is a fundamental technique in analytical chemistry.
-
pH Adjustment: The reaction can be used to adjust the pH of a solution. Adding HCl to a solution of Ca(OH)₂ will decrease the pH, making it less alkaline.
-
Industrial Applications: Calcium hydroxide is used in many industrial processes, including the production of cement, paper, and textiles. The reaction with acids, such as HCl, can be involved in various steps of these processes.
-
Wastewater Treatment: Neutralization reactions play a crucial role in wastewater treatment to adjust the pH of wastewater before discharge. Strong acids or bases in wastewater can be neutralized using the opposite type of compound.
Safety Precautions
Hydrochloric acid and calcium hydroxide are corrosive substances. When handling these chemicals, always wear appropriate personal protective equipment (PPE), such as safety goggles, gloves, and lab coats. Work in a well-ventilated area to avoid inhaling fumes. In case of accidental contact with skin or eyes, immediately flush with plenty of water and seek medical attention if necessary.
Conclusion
The balanced equation for the reaction between HCl and Ca(OH)₂, 2HCl + Ca(OH)₂ → CaCl₂ + 2H₂O, represents a fundamental acid-base neutralization reaction. Understanding this equation and the underlying principles of balancing chemical equations is crucial for various applications in chemistry and related fields. Always remember to prioritize safety when handling these chemicals. This reaction serves as a cornerstone for understanding stoichiometry, titrations, and the concepts of acids, bases, and salts. The comprehensive understanding gained from this balanced equation helps to build a strong foundation in chemistry and its wide array of applications. Further exploration into the reaction's thermodynamics and kinetics can provide an even more complete understanding of this important chemical process.
Latest Posts
Latest Posts
-
What Is 2 And 1 4 As An Improper Fraction
Apr 02, 2025
-
What Is 18 24 As A Percent
Apr 02, 2025
-
How Do You Graph X 6
Apr 02, 2025
-
2000 Mg Equals How Many G
Apr 02, 2025
-
How Many Electrons Can A Single Orbital Hold
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Hcl Ca Oh 2 Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
