How Many Atoms In Sulfuric Acid
listenit
Mar 31, 2025 · 7 min read
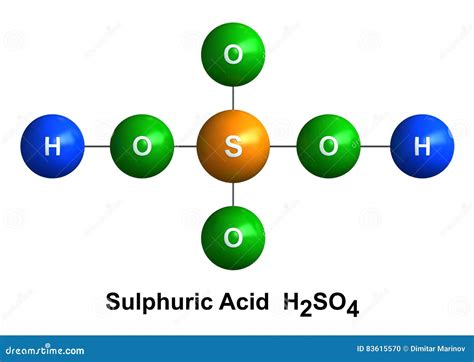
Table of Contents
Delving into the Atomic Composition of Sulfuric Acid: A Comprehensive Exploration
Sulfuric acid, with its potent corrosive properties and widespread industrial applications, is a chemical compound of significant interest. Understanding its atomic composition goes beyond a simple formula; it delves into the fundamental building blocks of matter and the intricacies of chemical bonding. This article will provide a comprehensive exploration of the number of atoms in a molecule of sulfuric acid, examining its chemical formula, structural arrangement, and the implications of its atomic composition for its properties and applications.
Understanding the Chemical Formula: H₂SO₄
The chemical formula for sulfuric acid is H₂SO₄. This formula concisely communicates the types and quantities of atoms present in a single molecule of the substance. Let's break it down:
-
H₂: This signifies the presence of two hydrogen atoms (H). Hydrogen, the simplest element, possesses one proton and one electron. Its relatively small size and single valence electron contribute significantly to sulfuric acid's reactivity.
-
S: This represents one sulfur atom (S). Sulfur, a nonmetal in the chalcogen group, plays a central role in determining sulfuric acid's structure and properties. Its ability to form multiple bonds is key to the molecule's stability and acidity.
-
O₄: This indicates the presence of four oxygen atoms (O). Oxygen, a highly electronegative element, is crucial in creating the strong covalent bonds within the sulfuric acid molecule. Its presence dictates much of the molecule's polarity and reactivity.
Therefore, a single molecule of sulfuric acid contains a total of seven atoms: two hydrogen atoms, one sulfur atom, and four oxygen atoms.
Exploring the Molecular Structure: Beyond the Formula
The chemical formula provides a quantitative description of the atoms, but it doesn't fully capture the spatial arrangement of these atoms. The molecular structure of sulfuric acid reveals a tetrahedral arrangement around the central sulfur atom.
-
Central Sulfur Atom: The sulfur atom resides at the center of the molecule, acting as the central hub for bonding. Its six valence electrons participate in forming covalent bonds with the surrounding oxygen atoms.
-
Oxygen Atoms: Four oxygen atoms are bonded to the central sulfur atom. Two of these oxygen atoms form double bonds with the sulfur atom (S=O), while the remaining two form single bonds (S-O). These single-bonded oxygen atoms are also bonded to hydrogen atoms, forming hydroxyl groups (-OH).
-
Hydrogen Atoms: The two hydrogen atoms are attached to the oxygen atoms, forming hydroxyl groups (-OH). The presence of these hydroxyl groups contributes to sulfuric acid's acidic nature, as they readily donate protons (H⁺) in aqueous solutions.
This tetrahedral geometry and the presence of both single and double bonds contribute to the molecule's stability and its unique reactivity.
The Significance of Atomic Composition in Sulfuric Acid's Properties
The specific number and arrangement of atoms in sulfuric acid directly influence its chemical and physical properties. Several key properties are intrinsically linked to its atomic composition:
-
High Acidity: The presence of two hydroxyl groups (-OH) bonded to oxygen atoms, which are in turn bonded to the sulfur atom, makes sulfuric acid a strong acid. These hydroxyl groups readily donate protons (H⁺) in aqueous solutions, resulting in a highly acidic nature. This high acidity underlies many of its industrial applications.
-
High Polarity: The electronegativity difference between sulfur, oxygen, and hydrogen leads to a highly polar molecule. The oxygen atoms pull electron density towards themselves, creating a partial negative charge (δ-) on the oxygen atoms and a partial positive charge (δ+) on the hydrogen atoms. This polarity enables sulfuric acid to dissolve many ionic compounds.
-
Strong Oxidizing Agent: At high concentrations, sulfuric acid can act as a strong oxidizing agent. This property stems from the sulfur atom's ability to accept electrons, reducing its oxidation state. This oxidizing capability is utilized in various industrial processes.
-
Dehydrating Agent: Sulfuric acid's strong affinity for water molecules makes it an effective dehydrating agent. It can remove water from other compounds, resulting in the formation of water and other products. This is exploited in several applications where water removal is necessary.
-
Viscosity: The strong intermolecular forces (hydrogen bonding and dipole-dipole interactions) arising from the polar nature of the molecule contribute to sulfuric acid's high viscosity. This makes it a thick, syrupy liquid at room temperature.
Industrial Applications: A Reflection of Atomic Composition
The unique properties of sulfuric acid, stemming directly from its atomic composition, have led to its widespread use in numerous industries:
-
Fertilizer Production: Sulfuric acid is a crucial component in the production of phosphate fertilizers, which are essential for agricultural productivity. Its role in converting phosphate rock into soluble phosphates demonstrates its chemical reactivity.
-
Petroleum Refining: In petroleum refining, sulfuric acid is used in various processes, including alkylation and the removal of impurities. Its ability to act as both an acid catalyst and an oxidizing agent makes it valuable in this industry.
-
Metal Processing: Sulfuric acid is used in the processing of various metals, including copper, nickel, and zinc. Its ability to dissolve metal oxides and other impurities is crucial in these processes.
-
Chemical Synthesis: Sulfuric acid serves as a catalyst and reagent in numerous chemical syntheses, including the production of dyes, pigments, and detergents. Its versatility and high reactivity are fundamental to its role in these applications.
-
Battery Production: Sulfuric acid is the electrolyte in lead-acid batteries, which power vehicles and other devices. Its high ionic conductivity is key to the proper functioning of these batteries.
Beyond the Single Molecule: Moles and Avogadro's Number
While we've focused on the number of atoms in a single molecule of sulfuric acid, it's important to consider the concept of moles and Avogadro's number when dealing with macroscopic quantities. A mole is a unit of measurement in chemistry that represents a specific number of entities (atoms, molecules, ions, etc.). This number is Avogadro's number, approximately 6.022 x 10²³.
One mole of sulfuric acid contains 6.022 x 10²³ molecules of H₂SO₄. To calculate the total number of atoms in one mole of sulfuric acid, we multiply Avogadro's number by the number of atoms in a single molecule:
(6.022 x 10²³ molecules/mol) x (7 atoms/molecule) = 4.215 x 10²⁴ atoms/mol
Therefore, one mole of sulfuric acid contains approximately 4.215 x 10²⁴ atoms. This illustrates the vast number of atoms involved in even relatively small amounts of this common chemical.
Isotopes and Atomic Mass: A Deeper Dive
The discussion so far has considered the most common isotopes of hydrogen, sulfur, and oxygen. However, each element has multiple isotopes, which differ in the number of neutrons in their nuclei. These isotopic variations subtly affect the average atomic mass of the elements and therefore the overall mass of a sulfuric acid molecule.
Taking into account the natural abundance of each isotope, the average atomic mass of hydrogen is approximately 1.008 amu, sulfur is approximately 32.06 amu, and oxygen is approximately 16.00 amu. The molecular weight of sulfuric acid can be calculated as follows:
2(1.008 amu) + 32.06 amu + 4(16.00 amu) ≈ 98.08 amu
This calculated molecular weight incorporates the slight mass differences due to isotopic variations.
Conclusion: A Fundamental Understanding
The seemingly simple chemical formula H₂SO₄ belies a complex interplay of atomic forces and interactions. Understanding the number of atoms in a sulfuric acid molecule – seven atoms in total, specifically two hydrogen, one sulfur, and four oxygen – provides a foundation for grasping its unique properties and diverse applications. The arrangement of these atoms, their bonding characteristics, and the resulting molecular structure dictate sulfuric acid’s strong acidity, high polarity, oxidizing potential, and dehydrating capabilities. This understanding extends beyond the single molecule to encompass molar quantities and the influence of isotopic variations. Ultimately, a deep understanding of sulfuric acid's atomic composition is crucial for appreciating its profound significance in various scientific and industrial contexts. The seemingly simple formula H₂SO₄ represents a wealth of chemical complexity and multifaceted utility.
Latest Posts
Latest Posts
-
Write A Balanced Overall Reaction From These Unbalanced Half Reactions
Apr 01, 2025
-
What Is The Reciprocal Of 1 25
Apr 01, 2025
-
Hcl Ca Oh 2 Balanced Equation
Apr 01, 2025
-
Is 6 7 A Rational Number
Apr 01, 2025
-
65 As A Fraction Simplest Form
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms In Sulfuric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
