How To Find Ph At The Equivalence Point
listenit
Mar 21, 2025 · 6 min read
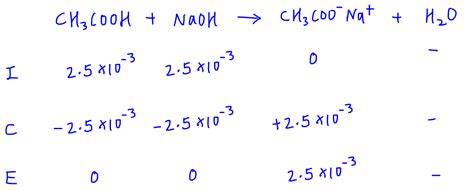
Table of Contents
How to Find pH at the Equivalence Point: A Comprehensive Guide
Determining the pH at the equivalence point of a titration is crucial in analytical chemistry. It provides valuable insights into the strength of the acid and base involved and allows for accurate quantitative analysis. This comprehensive guide will explore various methods for calculating the pH at the equivalence point, focusing on different types of titrations and providing practical examples to solidify your understanding.
Understanding the Equivalence Point
Before delving into the calculations, let's clarify the concept of the equivalence point. In a titration, the equivalence point is reached when the moles of titrant added are stoichiometrically equal to the moles of analyte present. This point signifies the complete neutralization of the analyte. It's important to distinguish the equivalence point from the endpoint, which is the point where the indicator changes color, signifying the approximate completion of the neutralization reaction. Ideally, the equivalence point and the endpoint should be very close to each other.
Calculating pH at the Equivalence Point: Different Titration Types
The method for calculating the pH at the equivalence point differs depending on the nature of the acid and base involved:
1. Strong Acid-Strong Base Titration
This is the simplest case. The reaction between a strong acid (e.g., HCl) and a strong base (e.g., NaOH) produces a neutral salt and water. At the equivalence point, the solution contains only the salt and water. Since neither the cation nor the anion of the salt undergoes hydrolysis (reaction with water to produce H⁺ or OH⁻ ions), the pH at the equivalence point is 7.
Example: Titration of 0.1 M HCl with 0.1 M NaOH. At the equivalence point, the pH will be 7.
2. Weak Acid-Strong Base Titration
This is more complex. The reaction of a weak acid (e.g., acetic acid, CH₃COOH) with a strong base (e.g., NaOH) produces a salt of the conjugate base (e.g., sodium acetate, CH₃COONa) and water. The conjugate base undergoes hydrolysis, producing hydroxide ions (OH⁻) and increasing the pH. To find the pH, we need to consider the hydrolysis of the conjugate base.
Calculation Steps:
-
Determine the concentration of the conjugate base: At the equivalence point, the moles of the conjugate base are equal to the initial moles of the weak acid. Use this to calculate the concentration of the conjugate base.
-
Calculate the Kb of the conjugate base: The Kb (base dissociation constant) is related to the Ka (acid dissociation constant) of the weak acid by the equation: Kw = Ka * Kb, where Kw is the ion product constant for water (1.0 x 10⁻¹⁴ at 25°C).
-
Use the Kb expression to find the hydroxide ion concentration [OH⁻]: Kb = [OH⁻][HA]/[A⁻] where [HA] is the concentration of the weak acid and [A⁻] is the concentration of the conjugate base. Since [OH⁻] ≈ [HA], we can simplify the equation.
-
Calculate the pOH: pOH = -log[OH⁻]
-
Calculate the pH: pH = 14 - pOH
Example: Titration of 0.1 M acetic acid (Ka = 1.8 x 10⁻⁵) with 0.1 M NaOH.
- First, determine the concentration of acetate ions at the equivalence point (which will be approximately 0.05M assuming equal volumes of acid and base).
- Calculate Kb: Kb = Kw/Ka = (1.0 x 10⁻¹⁴)/(1.8 x 10⁻⁵) ≈ 5.6 x 10⁻¹⁰
- Use the ICE table and Kb expression to solve for [OH⁻].
- Calculate pOH and then pH. The pH will be greater than 7.
3. Weak Base-Strong Acid Titration
This is analogous to the weak acid-strong base titration. The reaction of a weak base (e.g., ammonia, NH₃) with a strong acid (e.g., HCl) produces a salt of the conjugate acid (e.g., ammonium chloride, NH₄Cl) and water. The conjugate acid undergoes hydrolysis, producing hydronium ions (H₃O⁺) and decreasing the pH.
Calculation Steps: Follow similar steps as the weak acid-strong base titration, but use the Ka of the conjugate acid instead of the Kb of the conjugate base and solve for [H₃O⁺] instead of [OH⁻].
Example: Titration of 0.1 M ammonia (Kb = 1.8 x 10⁻⁵) with 0.1 M HCl. The pH will be less than 7 at the equivalence point.
4. Polyprotic Acid-Strong Base Titration
Polyprotic acids have multiple ionizable protons. Their titration curves show multiple equivalence points. Calculating the pH at each equivalence point requires considering the successive dissociation constants (Ka1, Ka2, etc.) and the hydrolysis of the conjugate bases. This involves multiple equilibrium calculations and can be quite complex. For example, a diprotic acid like sulfuric acid will have two equivalence points, each requiring separate calculations.
5. Using the Henderson-Hasselbalch Equation (Approximation)
The Henderson-Hasselbalch equation is a useful approximation for calculating the pH of a buffer solution, which exists near the equivalence point in weak acid-strong base and weak base-strong acid titrations, but not at the equivalence point itself. While it doesn't directly provide the pH at the equivalence point, understanding its application is crucial for understanding the titration curve's behavior around the equivalence point.
Factors Affecting pH at the Equivalence Point
Several factors can influence the pH at the equivalence point:
- Temperature: The Kw of water is temperature-dependent, impacting the pH calculations, especially for weak acid/base titrations.
- Ionic Strength: High ionic strength can influence activity coefficients and thus affect the equilibrium constants, leading to deviations from the calculated pH.
- Concentration of the acid and base: The concentration influences the degree of hydrolysis and hence the pH.
- Presence of other ions: The presence of other ions in the solution can influence the activity coefficients and the equilibrium, leading to deviations from the calculated pH.
Practical Applications and Significance
Determining the pH at the equivalence point is vital in various applications:
- Quantitative analysis: It allows for the precise determination of the concentration of an unknown acid or base.
- Determining the pKa of a weak acid or pKb of a weak base: The pH at the half-equivalence point (where half the analyte has been neutralized) is equal to the pKa (or pKb). This information is valuable in characterizing the acid or base.
- Quality control: It's crucial in various industrial processes to ensure the purity and quality of chemicals.
- Environmental monitoring: It plays a role in water quality analysis and environmental assessments.
Advanced Techniques and Considerations
For complex titrations, more advanced techniques and software may be necessary, such as iterative calculations or computer simulations. These approaches can handle the intricate equilibrium calculations involved in polyprotic acid titrations or titrations involving complex ions.
Conclusion
Calculating the pH at the equivalence point is a fundamental skill in analytical chemistry. While straightforward for strong acid-strong base titrations, it requires a thorough understanding of equilibrium and hydrolysis principles for other titration types. By mastering the techniques described in this guide, you can accurately determine the pH at the equivalence point, unlocking valuable insights into acid-base reactions and their applications in various fields. Remember to always consider the specific nature of the acid and base involved, and utilize appropriate calculation methods for accurate and meaningful results.
Latest Posts
Latest Posts
-
3 Main Ideas Of Cell Theory
Mar 28, 2025
-
What Are The Most Reactive Metals In The Periodic Table
Mar 28, 2025
-
Is Supports Combustion A Physical Or Chemical Property
Mar 28, 2025
-
What Is 6 Divided By 1 3
Mar 28, 2025
-
Add Acid To Water Or Water To Acid
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How To Find Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
