How To Find Neutrons On A Periodic Table
listenit
Mar 24, 2025 · 6 min read
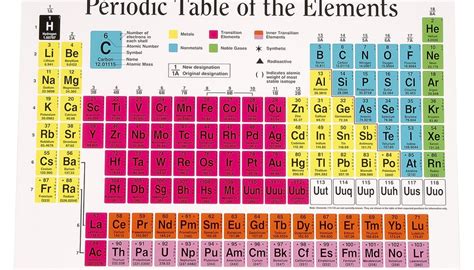
Table of Contents
How to Find Neutrons on a Periodic Table: A Comprehensive Guide
Finding the exact number of neutrons for an element using only a standard periodic table can be tricky. The periodic table provides the atomic number (number of protons) and the average atomic mass, but not the neutron count directly. This is because the number of neutrons in an element can vary, resulting in isotopes. This guide will break down how to understand what the periodic table does tell you, and how to then use that information to calculate the number of neutrons for a given isotope.
Understanding the Periodic Table's Limitations
The periodic table is a fantastic tool for organizing elements, showcasing their properties and relationships. However, it has inherent limitations when it comes to providing precise neutron counts. Here's why:
-
Atomic Number (Z): The periodic table clearly shows the atomic number (Z) for each element. This number represents the number of protons in the atom's nucleus. Since the number of protons defines the element itself (e.g., all atoms with 6 protons are carbon), this is a constant and readily available.
-
Average Atomic Mass (A): The periodic table also lists the average atomic mass (A) for each element. This is a weighted average of the masses of all naturally occurring isotopes of that element. This is where things get more complex.
-
Isotopes and Neutron Variation: Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. For example, carbon has two common isotopes: Carbon-12 (⁶¹²C) and Carbon-13 (⁶¹³C). Both have 6 protons, but Carbon-12 has 6 neutrons, and Carbon-13 has 7 neutrons. The average atomic mass reflects the abundance of each isotope in nature.
Therefore, the average atomic mass shown on the periodic table doesn't directly tell you the neutron count for any specific isotope of an element. It's a weighted average, obscuring the exact neutron number.
Calculating the Number of Neutrons: A Step-by-Step Process
To find the number of neutrons in a specific isotope, you need to know its mass number (A). The mass number is the total number of protons and neutrons in the nucleus. Once you have the mass number, the calculation is straightforward:
Number of Neutrons = Mass Number (A) - Atomic Number (Z)
Let's break this down step-by-step:
-
Identify the Element and its Atomic Number: Locate the element on the periodic table. Its atomic number (Z) is usually displayed above the element's symbol. For example, for Carbon (C), Z = 6.
-
Determine the Isotope's Mass Number: The mass number (A) of a specific isotope is usually written as a superscript to the left of the element's symbol. For instance, Carbon-14 is written as ¹⁴C. In this case, A = 14. You'll often find this information in scientific contexts or specialized resources; the periodic table itself doesn't list individual isotopes' mass numbers.
-
Perform the Calculation: Substitute the values of A and Z into the formula:
Number of Neutrons = A - Z
For ¹⁴C: Number of Neutrons = 14 - 6 = 8 neutrons.
Where to Find Isotopic Mass Numbers
Since the periodic table itself doesn't contain a complete list of isotopes for each element and their respective mass numbers, you'll need to consult other resources:
-
Chemistry Textbooks and Handbooks: These often include tables listing isotopes and their abundances.
-
Online Isotope Databases: Several reputable online databases maintain comprehensive information on isotopes, including their mass numbers and relative abundances. A simple online search for "isotope database" will yield many results. These databases provide far more information than what you find in a standard periodic table.
-
Scientific Literature: Research papers and scientific articles often include details on specific isotopes used in experiments.
Understanding Isotopic Abundance and Average Atomic Mass
The average atomic mass on the periodic table is not a direct measure of the number of neutrons in a single atom. Instead, it reflects the weighted average of the masses of all naturally occurring isotopes of the element. The calculation considers the mass of each isotope and its relative abundance in nature.
For example, let's consider Chlorine (Cl). Chlorine has two main isotopes: ³⁵Cl and ³⁷Cl. ³⁵Cl has a relative abundance of about 75% and ³⁷Cl has a relative abundance of about 25%. The average atomic mass is calculated by weighting the mass of each isotope by its abundance.
- ³⁵Cl mass ≈ 35 amu (atomic mass units)
- ³⁷Cl mass ≈ 37 amu
Average atomic mass of Chlorine ≈ (0.75 * 35) + (0.25 * 37) ≈ 35.5 amu
This average atomic mass (35.5 amu) is what is typically shown on the periodic table. It represents the average mass of a chlorine atom, considering the mixture of isotopes found in nature. It doesn't mean any single chlorine atom has 35.5 neutrons.
Advanced Concepts: Nuclear Stability and Isotope Decay
The number of neutrons in an isotope significantly impacts its nuclear stability. Certain neutron-to-proton ratios lead to more stable isotopes, while others are radioactive and undergo decay. Understanding isotope decay and the factors influencing it requires a deeper dive into nuclear physics.
-
Stable Isotopes: These isotopes have a neutron-to-proton ratio that makes them relatively stable and don't spontaneously decay.
-
Radioactive Isotopes: These isotopes have an unstable neutron-to-proton ratio, resulting in radioactive decay. They emit particles (alpha, beta, gamma) to achieve a more stable configuration.
-
Half-life: Radioactive isotopes decay at a specific rate, characterized by their half-life, the time it takes for half of the sample to decay.
Applications of Isotope Knowledge
Knowing the number of neutrons in an isotope has crucial applications in various fields:
-
Nuclear Medicine: Radioactive isotopes are used in diagnostic imaging (PET scans, etc.) and radiotherapy to treat cancer. The specific isotope's properties, determined partly by its neutron count, are essential for these applications.
-
Archaeology and Dating: Radiocarbon dating utilizes the decay of ¹⁴C to determine the age of ancient artifacts. Understanding the decay process is vital for accurate dating.
-
Nuclear Chemistry and Physics: Studies involving nuclear reactions and nuclear properties heavily rely on precise knowledge of isotope compositions and neutron numbers.
-
Material Science: The properties of materials can be significantly altered by the isotopic composition. This is crucial for designing materials with specific characteristics.
Conclusion
While the standard periodic table doesn't directly provide the number of neutrons for individual isotopes, it gives you the atomic number, essential for calculating neutron count once you know the isotope's mass number. Remember to consult additional resources like textbooks, databases, or scientific literature to find the mass numbers of specific isotopes to accurately determine their neutron count. This comprehensive understanding of atomic structure, isotopes, and their properties is vital in various scientific disciplines and practical applications. The periodic table serves as a foundation, but deeper exploration is needed for detailed isotopic information.
Latest Posts
Latest Posts
-
How Many Neutrons Are In C14
Mar 26, 2025
-
Geometric Mean Of 3 And 12
Mar 26, 2025
-
What Is Greater 3 4 Or 2 3
Mar 26, 2025
-
How Can An Igneous Rock Become A Metamorphic Rock
Mar 26, 2025
-
What Is The Gcf Of 28 And 24
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How To Find Neutrons On A Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
