How To Calculate The Molar Ratio
listenit
Mar 25, 2025 · 5 min read
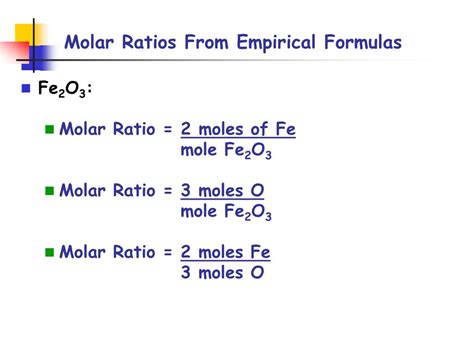
Table of Contents
How to Calculate the Molar Ratio: A Comprehensive Guide
Understanding molar ratios is fundamental to many areas of chemistry, from stoichiometry calculations to determining the limiting reactant in a chemical reaction. This comprehensive guide will delve into the intricacies of calculating molar ratios, providing you with a step-by-step approach, numerous examples, and helpful tips to master this crucial concept.
What is a Molar Ratio?
A molar ratio is the ratio of the amounts (in moles) of any two compounds involved in a chemical reaction. It's essentially a quantitative representation of the relative proportions of reactants and products in a balanced chemical equation. This ratio is crucial because it directly reflects the stoichiometry – the quantitative relationships between reactants and products in a chemical reaction. Understanding molar ratios allows you to:
- Predict the amount of product formed: Knowing the molar ratio lets you calculate how much product you'll get from a given amount of reactant.
- Determine the limiting reactant: By comparing molar ratios, you can identify the reactant that will be completely consumed first, limiting the amount of product formed.
- Solve stoichiometry problems: Molar ratios are the cornerstone of solving a wide range of stoichiometry problems.
How to Calculate Molar Ratios: A Step-by-Step Approach
Calculating molar ratios involves several key steps:
Step 1: Write and Balance the Chemical Equation
This is the most crucial first step. A balanced chemical equation provides the exact numerical relationship between reactants and products. For example, consider the reaction between hydrogen and oxygen to form water:
2H₂ + O₂ → 2H₂O
This equation tells us that two moles of hydrogen react with one mole of oxygen to produce two moles of water.
Step 2: Identify the Moles of Each Compound
Before calculating the molar ratio, you need to know the number of moles of each compound involved. Recall that the number of moles (n) is calculated using the formula:
n = mass (g) / molar mass (g/mol)
Where:
- n represents the number of moles
- mass is the mass of the substance in grams
- molar mass is the mass of one mole of the substance, found by adding up the atomic masses from the periodic table.
Step 3: Determine the Molar Ratio
Once you have the number of moles for each compound, you can calculate the molar ratio. The molar ratio is simply the ratio of the number of moles of one compound to the number of moles of another compound. The ratio is typically expressed as a fraction or a colon (:).
For example, in the balanced equation above:
- Molar ratio of H₂ to O₂: 2 mol H₂ / 1 mol O₂ = 2:1
- Molar ratio of H₂ to H₂O: 2 mol H₂ / 2 mol H₂O = 1:1
- Molar ratio of O₂ to H₂O: 1 mol O₂ / 2 mol H₂O = 1:2
Note: The molar ratio is always derived from the coefficients in the balanced chemical equation.
Examples of Molar Ratio Calculations
Let's work through a few examples to solidify your understanding:
Example 1: Reaction of Sodium and Chlorine
Consider the reaction of sodium (Na) and chlorine (Cl₂) to form sodium chloride (NaCl):
2Na + Cl₂ → 2NaCl
If you react 4 moles of sodium with 2 moles of chlorine, what is the molar ratio of sodium to chlorine?
- Moles of Na: 4 moles
- Moles of Cl₂: 2 moles
- Molar ratio of Na to Cl₂: 4 moles Na / 2 moles Cl₂ = 2:1
This ratio shows that sodium is present in a 2:1 ratio compared to chlorine, even though there are more moles of sodium available than chlorine.
Example 2: Determining the Limiting Reactant
Consider the reaction:
N₂ + 3H₂ → 2NH₃
If you have 5 moles of N₂ and 12 moles of H₂, which is the limiting reactant?
- Moles of N₂: 5 moles
- Moles of H₂: 12 moles
Based on the balanced equation, the molar ratio of N₂ to H₂ is 1:3. To determine the limiting reactant, we'll see how many moles of H₂ are needed to react completely with 5 moles of N₂:
5 moles N₂ * (3 moles H₂ / 1 mole N₂) = 15 moles H₂
Since you only have 12 moles of H₂, hydrogen is the limiting reactant.
Example 3: Calculating the Amount of Product
Using the same reaction as in Example 2:
N₂ + 3H₂ → 2NH₃
If you start with 2 moles of N₂ and excess H₂, how many moles of NH₃ will be produced?
The molar ratio of N₂ to NH₃ is 1:2. Therefore:
2 moles N₂ * (2 moles NH₃ / 1 mole N₂) = 4 moles NH₃
4 moles of NH₃ will be produced.
Advanced Applications of Molar Ratios
Beyond basic stoichiometry, molar ratios are applied in various advanced chemical concepts:
- Acid-base titrations: Molar ratios are used to determine the concentration of an unknown acid or base by titrating it with a solution of known concentration.
- Solubility product calculations: The molar ratios of ions in a saturated solution are used to calculate the solubility product constant (Ksp).
- Equilibrium calculations: Molar ratios are used to express the equilibrium constant (Kc) for reversible reactions.
- Gas stoichiometry: Molar ratios can be used to calculate volumes of gases involved in reactions using the Ideal Gas Law.
Tips and Tricks for Mastering Molar Ratio Calculations
- Always balance the chemical equation first: An unbalanced equation will lead to incorrect molar ratios and flawed calculations.
- Use units consistently: Make sure you use consistent units (moles, grams, liters, etc.) throughout your calculations.
- Double-check your work: Mistakes in calculation are common. Review your work carefully and ensure your answer makes chemical sense.
- Practice regularly: The best way to master molar ratio calculations is through consistent practice. Work through various examples and problems to build your confidence and understanding.
- Seek help when needed: Don't hesitate to ask your teacher, professor, or tutor for assistance if you're struggling.
Conclusion
Understanding and calculating molar ratios is a fundamental skill in chemistry. By following the steps outlined in this guide and practicing regularly, you can develop a strong grasp of this essential concept. Remember that mastering molar ratios opens the door to solving a vast array of stoichiometry problems and unlocking a deeper understanding of chemical reactions. This skill will serve you well throughout your chemistry studies and beyond. Remember to always double-check your work and ensure your units are consistent! With practice, you'll become proficient in calculating molar ratios and applying this knowledge to solve various chemical problems.
Latest Posts
Latest Posts
-
What Is The Formula For The Compound Magnesium Oxide
Mar 28, 2025
-
What Is The Correct Formula For Calcium Oxide
Mar 28, 2025
-
What Is The Si Base Unit Of Length
Mar 28, 2025
-
What Is The Oxidation State Of Each Element In Coh2
Mar 28, 2025
-
How Many Valence Electrons Are In Boron
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate The Molar Ratio . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
