How Many Valence Electrons In Sodium
listenit
Mar 22, 2025 · 5 min read
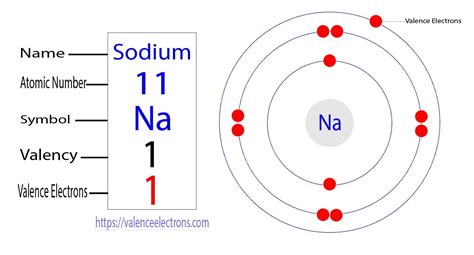
Table of Contents
How Many Valence Electrons Does Sodium Have? A Deep Dive into Sodium's Electronic Structure
Sodium, a ubiquitous element found in table salt and essential for human life, holds a fascinating position in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is crucial for comprehending its chemical behavior and reactivity. This comprehensive guide delves deep into the question: How many valence electrons does sodium have? We'll explore the concept of valence electrons, examine sodium's electron configuration, and discuss its implications for sodium's properties and reactions.
Understanding Valence Electrons: The Key to Reactivity
Before diving into sodium's specifics, let's establish a firm grasp on the concept of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the furthest from the nucleus and are therefore most loosely bound. This loose binding makes them the primary participants in chemical bonding. The number of valence electrons determines an atom's reactivity and how it will interact with other atoms to form chemical compounds. Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons to achieve a full outer shell, a state often referred to as the octet rule (eight electrons in the outer shell, except for hydrogen and helium which aim for two).
Sodium's Electron Configuration: Unveiling the Outermost Shell
Sodium (Na) has an atomic number of 11, meaning it possesses 11 protons and 11 electrons in a neutral atom. To understand the distribution of these electrons, we examine sodium's electron configuration. The electron configuration describes the arrangement of electrons in different energy levels or shells within an atom. For sodium, the electron configuration is 1s²2s²2p⁶3s¹.
Let's break this down:
- 1s²: This indicates two electrons in the first energy level (shell), specifically in the s subshell.
- 2s²: Two electrons in the second energy level, s subshell.
- 2p⁶: Six electrons in the second energy level, p subshell.
- 3s¹: One electron in the third energy level, s subshell.
The outermost shell of sodium is the third energy level (n=3). This shell contains only one electron in the 3s subshell.
The Answer: Sodium Possesses One Valence Electron
Therefore, based on its electron configuration, sodium has only one valence electron. This single valence electron is the key to understanding sodium's chemical behavior. Because it only needs to lose one electron to achieve a stable octet (matching the electron configuration of the noble gas Neon), sodium readily participates in ionic bonding.
Implications of Sodium's Single Valence Electron: Reactivity and Bonding
The presence of a single valence electron has profound consequences for sodium's chemical properties:
1. Highly Reactive Metal:
Sodium is a highly reactive alkali metal. Its eagerness to lose its single valence electron to achieve a stable octet makes it readily react with many other elements. This reactivity is why sodium is always found in compounds in nature, never as a free element.
2. Ionic Bonding:
Because losing one electron is energetically favorable for sodium, it typically forms ionic bonds. In ionic bonding, sodium readily donates its valence electron to a more electronegative atom, such as chlorine (Cl). This transfer of electrons creates positively charged sodium ions (Na⁺) and negatively charged ions of the other element. The electrostatic attraction between these oppositely charged ions constitutes the ionic bond. The classic example is the formation of sodium chloride (NaCl), common table salt, through the reaction of sodium and chlorine.
3. Oxidation State:
The single valence electron means sodium typically exhibits an oxidation state of +1. The oxidation state represents the apparent charge of an atom in a compound. Since sodium loses one electron, its charge becomes +1.
4. Electrical Conductivity:
The loosely held valence electron contributes to sodium's excellent electrical conductivity. The electron can move freely within the metallic lattice, facilitating the flow of electric current.
5. Reducing Agent:
Due to its tendency to lose an electron, sodium acts as a powerful reducing agent. A reducing agent donates electrons to other substances, causing them to be reduced (gain electrons). This property makes sodium useful in various chemical reactions and industrial processes.
Sodium's Role in Biological Systems: The Importance of a Single Valence Electron
The unique electronic structure of sodium, specifically its single valence electron, plays a crucial role in biological systems. Sodium ions (Na⁺) are essential for various biological processes, including:
-
Nerve Impulse Transmission: The movement of sodium ions across cell membranes is fundamental to nerve impulse transmission. Changes in sodium ion concentration trigger electrical signals that allow the nervous system to function.
-
Muscle Contraction: Sodium ions are involved in muscle contraction, enabling movement.
-
Fluid Balance: Sodium ions help regulate fluid balance within the body, maintaining proper hydration and blood pressure.
-
Nutrient Absorption: Sodium ions facilitate the absorption of nutrients from the digestive tract.
Beyond Sodium: Valence Electrons and the Periodic Table
Understanding the concept of valence electrons and their influence on chemical behavior extends far beyond sodium. The number of valence electrons is predictable based on an element's position in the periodic table. Elements within the same group (vertical column) share the same number of valence electrons, resulting in similar chemical properties. For example, all alkali metals (like lithium, potassium, rubidium, etc.) possess one valence electron, exhibiting similar reactivity to sodium.
This predictability is a powerful tool in chemistry, allowing scientists to anticipate the behavior of elements and predict the formation of compounds.
Conclusion: The Significance of Sodium's Single Valence Electron
The simple answer to "How many valence electrons does sodium have?" is one. However, this seemingly simple answer unlocks a deeper understanding of sodium's unique properties, reactivity, and its vital role in both chemical reactions and biological processes. The single valence electron dictates sodium's tendency to form ionic bonds, its role as a reducing agent, and its crucial involvement in biological functions. Understanding valence electrons is fundamental to grasping the principles of chemical bonding and the behavior of elements in general. The case of sodium serves as a perfect illustration of how a single electron can have such a profound impact on the world around us.
Latest Posts
Latest Posts
-
How Do You Factor X 2
Mar 22, 2025
-
Geometric Mean Of 5 And 15
Mar 22, 2025
-
Least Common Factor Of 3 And 8
Mar 22, 2025
-
What Is The Square Root Of 31
Mar 22, 2025
-
Graph The Parabola Y 3x 2
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
