How Many Valence Electrons Are In Ca+
listenit
Mar 25, 2025 · 5 min read
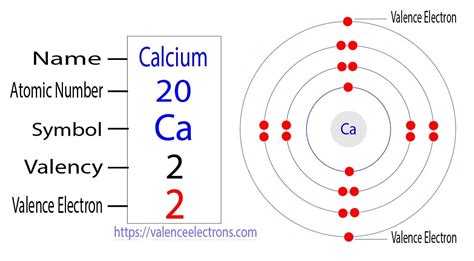
Table of Contents
How Many Valence Electrons are in Ca⁺? Understanding Calcium's Ions
Determining the number of valence electrons in an ion like Ca⁺ requires understanding the basics of electron configuration and how ionization affects an atom's outermost shell. This article will delve into the specifics of calcium (Ca) and its ion, Ca⁺, explaining the concept of valence electrons, electron configuration, and ionization. We will also explore the implications of this knowledge in various chemical contexts.
Understanding Valence Electrons
Valence electrons are the electrons located in the outermost shell of an atom. These electrons are crucial because they are the ones involved in chemical bonding. The number of valence electrons an atom possesses dictates its reactivity and the types of bonds it can form (ionic, covalent, metallic). Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons to obtain a full outer shell, usually resembling the electron configuration of a noble gas.
Calcium's Electron Configuration
Calcium (Ca) is an alkaline earth metal with an atomic number of 20. This means a neutral calcium atom contains 20 protons and 20 electrons. To determine the number of valence electrons, we need to understand its electron configuration. Following the Aufbau principle and Hund's rule, the electron configuration of a neutral calcium atom is:
1s²2s²2p⁶3s²3p⁶4s²
This configuration shows the distribution of electrons across different energy levels (shells). The outermost shell is the 4s shell, containing two electrons. Therefore, a neutral calcium atom has two valence electrons.
Ionization and Ca⁺
Ionization is the process by which an atom gains or loses electrons, resulting in the formation of an ion. When an atom loses electrons, it becomes a positively charged cation. Conversely, when it gains electrons, it becomes a negatively charged anion.
Calcium readily loses electrons to achieve a stable octet (a full outer shell). It tends to lose its two valence electrons in the 4s orbital, becoming a +2 ion (Ca²⁺). However, the question asks about Ca⁺, a calcium ion with only one electron removed.
The formation of Ca⁺ involves the loss of one electron from the 4s orbital. This means the electron configuration of Ca⁺ becomes:
1s²2s²2p⁶3s²3p⁶4s¹
Because the 4s orbital still holds one electron, Ca⁺ has one valence electron.
Implications of Ca⁺ Having One Valence Electron
While Ca²⁺ is the more common and stable ion for calcium, understanding the properties of Ca⁺ is crucial for certain chemical reactions and theoretical considerations. Its presence may be fleeting in many reactions. The single valence electron in Ca⁺ makes it highly reactive, readily participating in chemical reactions to achieve a more stable state, likely by losing the remaining electron to form the divalent Ca²⁺ ion.
The reactivity difference between Ca⁺ and Ca²⁺ highlights the significance of understanding ionization and valence electron count in predicting chemical behavior. While the divalent cation (Ca²⁺) is much more stable and commonly observed, the transient existence of Ca⁺ can offer valuable insights into the reaction mechanisms and energetics.
Comparing Ca, Ca⁺, and Ca²⁺
Let's summarize the key differences between neutral calcium and its ions:
| Species | Number of Protons | Number of Electrons | Valence Electrons | Electron Configuration | Stability |
|---|---|---|---|---|---|
| Ca | 20 | 20 | 2 | 1s²2s²2p⁶3s²3p⁶4s² | Relatively low |
| Ca⁺ | 20 | 19 | 1 | 1s²2s²2p⁶3s²3p⁶4s¹ | Low |
| Ca²⁺ | 20 | 18 | 0 | 1s²2s²2p⁶3s²3p⁶ | High (isoelectronic with Argon) |
This table clearly demonstrates the impact of ionization on the number of valence electrons and overall stability. The loss of each electron significantly alters the chemical properties of calcium.
Practical Applications and Further Considerations
While Ca⁺ might not be as prevalent as Ca²⁺ in typical chemical scenarios, its existence and properties have implications in various areas, such as:
- Spectroscopy: Studying the emission spectrum of calcium ions, including Ca⁺, provides information about energy levels and electron transitions within the ion. This is valuable for analytical chemistry and astrophysics.
- Plasma physics: In high-energy environments like plasmas, Ca⁺ ions can be significant components. Understanding their behavior is critical for controlling and utilizing plasma technologies.
- Theoretical Chemistry: Computational studies and theoretical modeling often explore the properties of less common ionic states, including Ca⁺, to understand fundamental chemical processes and reaction dynamics.
- Surface Science: Investigating the interaction of calcium with surfaces often involves considering different ionization states, including Ca⁺, and how these states affect surface reactivity and catalysis.
Conclusion: The Significance of Valence Electrons and Ionization
The number of valence electrons significantly influences an atom's chemical behavior. While a neutral calcium atom has two valence electrons, the formation of Ca⁺ results in a single valence electron. Understanding this difference is essential for predicting the reactivity and chemical properties of both neutral and ionized calcium. Although Ca²⁺ is the more prevalent and stable ion, recognizing the existence and properties of Ca⁺ enriches our comprehension of calcium's behavior under various conditions and its applications in different scientific fields. This underscores the importance of meticulously analyzing electronic configurations and the impact of ionization on the chemical characteristics of elements. The single valence electron in Ca⁺ significantly impacts its reactivity and overall stability, highlighting the importance of understanding these fundamental concepts in chemistry.
Latest Posts
Latest Posts
-
A Rhombus Is A Regular Polygon
Mar 28, 2025
-
What Muscle Subdivides The Ventral Body Cavity
Mar 28, 2025
-
What Are Three Main Ideas Of The Cell Theory
Mar 28, 2025
-
Hcl Ba Oh 2 Balanced Equation
Mar 28, 2025
-
7x 2y 13 X 2y 11
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Ca+ . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
