How Many Pi And Sigma Bonds In A Triple Bond
listenit
Mar 21, 2025 · 5 min read
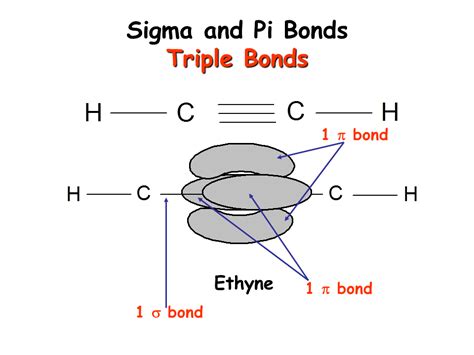
Table of Contents
How Many Pi and Sigma Bonds in a Triple Bond? A Deep Dive into Chemical Bonding
Understanding chemical bonding is fundamental to grasping the behavior of molecules. One of the key concepts within this field is the distinction between sigma (σ) and pi (π) bonds, particularly within the context of multiple bonds like double and triple bonds. This article delves into the intricacies of triple bonds, specifically addressing the question: How many pi and sigma bonds are in a triple bond? We'll explore the underlying principles of orbital hybridization and the formation of these bonds, providing a comprehensive understanding for students and enthusiasts alike.
The Fundamentals: Sigma and Pi Bonds
Before we dive into triple bonds, let's establish a firm understanding of sigma and pi bonds. These are two types of covalent bonds, which are formed by the sharing of electron pairs between atoms. The difference lies in the way the electron orbitals overlap:
Sigma (σ) Bonds
- Formation: A sigma bond is formed by the head-on overlap of atomic orbitals. This means the electron density is concentrated along the internuclear axis – the imaginary line connecting the centers of the two bonded atoms.
- Strength: Sigma bonds are generally stronger than pi bonds because of this direct overlap. They are the primary bond in a single bond.
- Rotation: Sigma bonds allow for free rotation around the bond axis.
Pi (π) Bonds
- Formation: A pi bond is formed by the sideways overlap of atomic orbitals, typically p orbitals. The electron density is concentrated above and below the internuclear axis.
- Strength: Pi bonds are generally weaker than sigma bonds due to less effective overlap.
- Rotation: Pi bonds restrict rotation around the bond axis. Breaking a pi bond is required for rotation to occur.
Delving into Double and Triple Bonds
Now let's consider how sigma and pi bonds combine to form double and triple bonds:
Double Bonds
A double bond consists of one sigma bond and one pi bond. The sigma bond forms initially, providing the primary link between the atoms. The additional pi bond arises from the sideways overlap of unhybridized p orbitals, strengthening the bond and influencing its properties. Think of ethene (C₂H₄) as a classic example.
Triple Bonds
Finally, we arrive at the core question of this article: How many pi and sigma bonds are in a triple bond? A triple bond comprises one sigma bond and two pi bonds. The formation proceeds in a similar manner to a double bond. The sigma bond forms first through head-on overlap. Then, two pi bonds are formed by the sideways overlap of two sets of unhybridized p orbitals. This results in a strong and rigid bond, limiting rotation. A prime example is the nitrogen molecule (N₂) where the strong triple bond contributes to its inert nature.
Orbital Hybridization and Bond Formation: A Deeper Look
To fully appreciate the formation of triple bonds, understanding orbital hybridization is crucial. Hybridization is the process of mixing atomic orbitals to form new hybrid orbitals with different shapes and energies. Let's examine the process with the example of ethyne (acetylene, C₂H₂), which contains a carbon-carbon triple bond.
Each carbon atom in ethyne is sp hybridized. This means one s orbital and one p orbital combine to form two sp hybrid orbitals, while the remaining two p orbitals remain unhybridized.
- Sigma Bond Formation: The two sp hybrid orbitals on each carbon atom overlap head-on to form a strong sigma bond between the carbon atoms. Each sp hybrid orbital also forms a sigma bond with a hydrogen atom.
- Pi Bond Formation: The two unhybridized p orbitals on each carbon atom undergo sideways overlap, forming two pi bonds. These pi bonds lie perpendicular to each other and to the internuclear axis of the sigma bond.
This arrangement results in a linear molecule with a strong, short carbon-carbon triple bond.
The Significance of Triple Bonds in Chemistry
Triple bonds, due to their unique structure and properties, play crucial roles in various chemical contexts:
- Stability: The strong bond strength of triple bonds contributes to the stability of molecules containing them. For instance, the nitrogen molecule (N₂) with its triple bond is remarkably stable and unreactive under normal conditions.
- Reactivity: While strong, triple bonds are also reactive, undergoing various addition reactions where the pi bonds are broken. This reactivity is exploited in many organic chemical syntheses.
- Structure and Functionality: The rigidity imparted by the triple bond influences the three-dimensional structure of molecules and affects their biological activity.
- Industrial Applications: Many industrially important compounds contain triple bonds, serving as building blocks for diverse applications ranging from plastics and polymers to pharmaceuticals and agrochemicals.
Common Examples of Triple Bonds
Several molecules showcase the presence of triple bonds, each contributing uniquely to their properties:
- Acetylene (Ethyne, C₂H₂): The simplest example, showcasing the fundamental features of a carbon-carbon triple bond.
- Nitrogen (N₂): A diatomic molecule with an extremely strong triple bond, explaining its inertness.
- Nitriles (RC≡N): Organic compounds containing a carbon-nitrogen triple bond, possessing unique chemical reactivity.
- Cyanides (CN⁻): An anion featuring a carbon-nitrogen triple bond, highly toxic due to its interaction with cellular processes.
- Carbon Monoxide (CO): Possesses a strong carbon-oxygen triple bond, with significant biological and industrial importance.
Beyond the Basics: Advanced Concepts
For those seeking a deeper understanding, we can explore some more advanced aspects:
- Bond Length and Bond Order: Triple bonds have the shortest bond length and highest bond order (3) compared to single (1) and double (2) bonds, reflecting the strength of the interaction.
- Bond Energy: The bond energy of a triple bond is significantly higher than that of a double or single bond, requiring more energy to break.
- Molecular Orbital Theory: A more advanced theoretical framework explains bond formation and properties based on the combination of atomic orbitals to form molecular orbitals.
- Spectroscopic Techniques: Techniques like infrared (IR) and Raman spectroscopy are useful in identifying the presence of triple bonds based on characteristic vibrational frequencies.
Conclusion
In conclusion, a triple bond consists of one sigma bond and two pi bonds. This unique combination of bonds results in a strong, short, and rigid linkage between atoms, significantly influencing the properties and reactivity of molecules containing them. Understanding the fundamental principles of sigma and pi bonds, as well as orbital hybridization, is essential for comprehending the behavior of molecules with triple bonds, providing valuable insights into their roles in various chemical and biological systems. This knowledge is crucial across diverse disciplines, underscoring the importance of this seemingly simple yet fundamentally significant aspect of chemical bonding.
Latest Posts
Latest Posts
-
When Gas Exerts Pressure On Its Container The Pressure Is
Mar 27, 2025
-
X 3 2 X 1 2
Mar 27, 2025
-
60 Of What Number Is 20
Mar 27, 2025
-
Graph The Linear Equation X 4
Mar 27, 2025
-
What Are Two Functional Groups Found In Amino Acids
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about How Many Pi And Sigma Bonds In A Triple Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
