How Many Neutrons Are In Carbon 12
listenit
Mar 24, 2025 · 5 min read
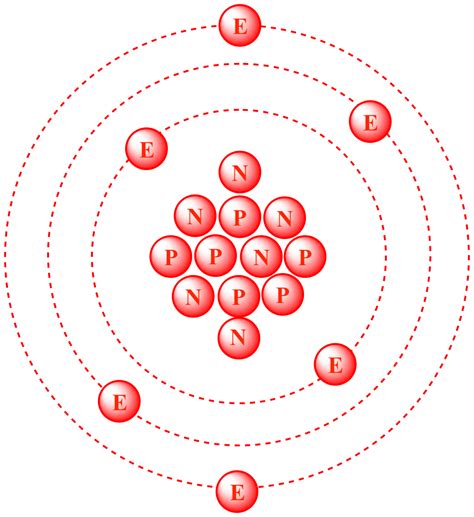
Table of Contents
- How Many Neutrons Are In Carbon 12
- Table of Contents
- How Many Neutrons are in Carbon-12? A Deep Dive into Isotopes and Nuclear Structure
- Understanding Atomic Structure: Protons, Neutrons, and Electrons
- Isotopes: Variations on a Theme
- Carbon-12: The Standard
- The Significance of Carbon-12
- Other Carbon Isotopes: Exploring Variations
- Nuclear Forces and Stability
- Applications of Isotope Analysis
- Beyond Carbon: Isotopes in Other Elements
- Conclusion: A Deeper Understanding of Carbon-12 and Beyond
- Latest Posts
- Latest Posts
- Related Post
How Many Neutrons are in Carbon-12? A Deep Dive into Isotopes and Nuclear Structure
The question, "How many neutrons are in carbon-12?" seems deceptively simple. However, understanding the answer requires delving into the fascinating world of isotopes, atomic structure, and nuclear physics. This comprehensive guide will not only answer that question directly but also explore the broader context of atomic nuclei and their significance in various scientific fields.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we tackle the specifics of carbon-12, let's establish a foundational understanding of atomic structure. Every atom consists of three fundamental subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element; all carbon atoms, for example, have six protons.
- Neutrons: Neutrally charged particles also residing in the nucleus. Unlike protons, the number of neutrons can vary within the same element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons typically equals the number of protons in a neutral atom.
Isotopes: Variations on a Theme
The term "isotope" refers to atoms of the same element that possess the same number of protons but differ in the number of neutrons. This difference in neutron count affects the atom's mass but not its chemical properties. Isotopes are often identified using a notation like this: <sup>A</sup>X<sub>Z</sub>, where:
- X represents the element's chemical symbol (e.g., C for carbon).
- Z represents the atomic number (number of protons).
- A represents the mass number (total number of protons and neutrons).
Carbon-12: The Standard
Carbon-12 (<sup>12</sup>C<sub>6</sub>) is the most abundant and stable isotope of carbon. Its notation tells us:
- Z = 6: It has six protons, defining it as carbon.
- A = 12: It has a mass number of 12.
Therefore, to determine the number of neutrons, we simply subtract the number of protons (Z) from the mass number (A):
Number of neutrons = A - Z = 12 - 6 = 6
Consequently, carbon-12 contains six neutrons.
The Significance of Carbon-12
Carbon-12 holds a special place in science due to its role as the basis for the unified atomic mass unit (amu). One amu is defined as exactly 1/12 the mass of a single carbon-12 atom. This standardized unit is crucial for expressing the relative masses of other atoms and molecules.
Other Carbon Isotopes: Exploring Variations
While carbon-12 is the most common isotope, carbon exists in other isotopic forms, including:
-
Carbon-13 (
<sup>13</sup>C<sub>6</sub>): This stable isotope contains seven neutrons (13 - 6 = 7). It's less abundant than carbon-12 but plays a role in various scientific applications, such as carbon dating and nuclear magnetic resonance (NMR) spectroscopy. -
Carbon-14 (
<sup>14</sup>C<sub>6</sub>): This isotope, with eight neutrons (14 - 6 = 8), is radioactive and has a half-life of approximately 5,730 years. Its radioactive decay is the basis for radiocarbon dating, a technique used to determine the age of organic materials. The presence and ratio of carbon-14 to carbon-12 helps to establish the age of materials.
The differing neutron counts in these isotopes lead to variations in their stability and applications. The additional neutrons alter the nuclear binding energy, influencing the stability and propensity for decay in radioactive isotopes.
Nuclear Forces and Stability
The stability of an atomic nucleus is governed by the interplay of strong nuclear forces and electromagnetic forces. Strong nuclear forces are responsible for binding protons and neutrons together within the nucleus, overcoming the repulsive electromagnetic forces between positively charged protons. The neutron-to-proton ratio is a crucial factor determining nuclear stability. For lighter elements, a ratio close to 1:1 often results in stable isotopes, as seen in carbon-12. However, as the atomic number increases, a higher neutron-to-proton ratio is required for stability.
Applications of Isotope Analysis
The ability to identify and quantify different isotopes has significant applications across diverse fields:
-
Geochemistry: Isotope ratios are used to trace the origin and movement of materials in geological processes. Analyzing the isotopic composition of rocks and minerals helps scientists to unravel Earth's history.
-
Environmental Science: Isotope analysis is used to track pollutants and understand environmental processes such as water cycling and nutrient flow. Isotopic tracers also assist in analyzing the impact of environmental changes.
-
Forensic Science: Isotopic analysis helps in identifying the origin of materials found at crime scenes. For example, the isotopic composition of drugs or other substances can help trace their sources and distribution networks.
-
Medicine: Radioisotopes are used in medical imaging and treatment. Positron emission tomography (PET) scans, for instance, utilize radioisotopes to visualize metabolic processes within the body.
-
Archaeology: Radiocarbon dating, using carbon-14, allows archaeologists to date organic materials and artifacts, providing crucial insights into past human civilizations.
Beyond Carbon: Isotopes in Other Elements
The concept of isotopes extends far beyond carbon. Most elements exist as a mixture of isotopes, each with a distinct number of neutrons. Understanding isotopic variations is vital for comprehending the behavior and properties of elements across the periodic table.
Conclusion: A Deeper Understanding of Carbon-12 and Beyond
In summary, carbon-12 contains six neutrons, a fact underpinned by our understanding of atomic structure and isotopic variations. The significance of carbon-12 extends beyond its simple neutron count, playing a pivotal role as the standard for atomic mass units and influencing numerous scientific applications. This exploration of carbon-12, however, serves as a gateway to comprehending the broader world of isotopes, their properties, and the profound impact they have on various scientific disciplines. The interplay of protons, neutrons, and their influence on nuclear stability remains a captivating area of scientific study, continuously revealing new insights into the fundamental building blocks of matter and the universe itself. The continued study and refinement of isotope analysis methods will undoubtedly lead to further advancements in diverse fields, from understanding the Earth's past to developing innovative medical technologies.
Latest Posts
Latest Posts
-
What Does Atomic Number Of An Element Represent
Mar 26, 2025
-
What Is The Electron Configuration For Chlorine
Mar 26, 2025
-
A Cyclist Accelerates From 0m S To 8
Mar 26, 2025
-
What Is The Conjugate Acid Of H2so4
Mar 26, 2025
-
How Much Is One Degree Celsius
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Are In Carbon 12 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
