How Many Electrons Can The 3rd Shell Hold
listenit
Apr 01, 2025 · 6 min read
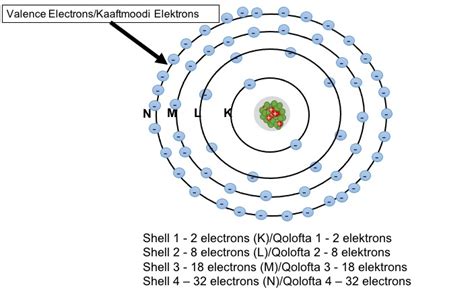
Table of Contents
How Many Electrons Can the 3rd Shell Hold? A Deep Dive into Electron Configuration
Understanding electron shells and their capacity is fundamental to grasping the behavior of atoms and the periodic table. This comprehensive guide delves into the specifics of the third electron shell, exploring its capacity, the sub-shells it contains, and the implications for chemical properties. We'll also touch upon the underlying principles of quantum mechanics that govern electron arrangement.
The Basics of Electron Shells and Subshells
Before diving into the third shell, let's review the fundamental concepts. Electrons, negatively charged particles, orbit the nucleus of an atom. They don't orbit in simple, predictable paths like planets around the sun, but rather exist in regions of space called electron shells or energy levels. These shells are arranged concentrically around the nucleus, with each shell representing a distinct energy level. The closer a shell is to the nucleus, the lower its energy.
Each shell isn't a uniform space; instead, it's comprised of subshells, denoted by the letters s, p, d, and f. These subshells differ in their shape and energy levels within a given shell. The number of subshells within a shell increases as the shell number increases.
- s subshell: Holds a maximum of 2 electrons and has a spherical shape.
- p subshell: Holds a maximum of 6 electrons and has a dumbbell shape.
- d subshell: Holds a maximum of 10 electrons and has more complex shapes.
- f subshell: Holds a maximum of 14 electrons and possesses even more intricate shapes.
The number of electrons a shell can hold is determined by the formula 2n², where 'n' is the principal quantum number (shell number).
The Third Electron Shell: Capacity and Subshells
Now, let's focus on the third electron shell (n=3). Using the formula 2n², we can calculate its maximum electron capacity: 2(3)² = 18 electrons. This means the third shell can hold a maximum of 18 electrons.
However, understanding how it holds these 18 electrons requires examining its subshells:
- 3s subshell: This subshell can hold a maximum of 2 electrons.
- 3p subshell: This subshell can hold a maximum of 6 electrons.
- 3d subshell: This subshell can hold a maximum of 10 electrons.
Therefore, the total capacity of the third shell (3s + 3p + 3d) is 2 + 6 + 10 = 18 electrons. This perfectly aligns with our earlier calculation using the 2n² formula.
Electron Configuration and the Third Shell
The arrangement of electrons within an atom's shells and subshells is known as its electron configuration. This configuration dictates an atom's chemical properties and reactivity. Electrons fill shells and subshells according to the Aufbau principle, which states that electrons occupy the lowest energy levels first. This means the 3s subshell fills before the 3p, and the 3p fills before the 3d.
For example, consider the element chlorine (Cl), which has 17 electrons. Its electron configuration is 1s²2s²2p⁶3s²3p⁵. Notice how the 3s subshell is filled before the 3p subshell, and the third shell is not completely filled. Similarly, consider iron (Fe), with 26 electrons: 1s²2s²2p⁶3s²3p⁶4s²3d⁶. Note that the 4s subshell fills before the 3d subshell, a slight deviation from the simplistic filling order explained above and related to the subtle energy differences between the subshells. This demonstrates that while the third shell can hold 18 electrons, it's not always fully occupied in every atom.
The Significance of the Third Shell's Electron Capacity
The third shell's ability to accommodate 18 electrons has significant consequences for the properties of elements:
-
Chemical Reactivity: Elements with partially filled third shells tend to be more reactive than those with completely filled or empty shells. This is because atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing electrons.
-
Periodic Trends: The periodic table organizes elements based on their electron configurations. The repeating patterns of chemical and physical properties observed across periods (rows) are directly related to the filling of electron shells and subshells, including the third shell.
-
Bonding: The number of electrons in the outermost shell (valence electrons) dictates how an atom bonds with other atoms. For elements with valence electrons in the third shell, the bonding behavior will reflect the number and arrangement of these electrons.
-
Metallic Character: Many transition metals, which possess partially filled 3d orbitals, exhibit characteristic metallic properties such as high electrical and thermal conductivity and malleability.
Quantum Mechanics and Electron Arrangement
The precise arrangement of electrons within the third shell (and other shells) is governed by the principles of quantum mechanics. These principles dictate that electrons don't follow simple, predictable paths but rather occupy regions of space called atomic orbitals, each capable of holding a maximum of two electrons with opposite spins (Pauli Exclusion Principle). The shapes and energy levels of these orbitals determine the subshells (s, p, d, f) within each shell.
The quantum mechanical model allows us to predict the electron configuration of atoms with greater accuracy than simpler models. It also explains the subtle differences in energy levels between subshells within the same shell, which influences the order in which subshells fill.
Beyond the Third Shell: Implications for Larger Atoms
As we move to higher shells (n=4, 5, 6, etc.), the number of subshells increases, leading to even more complex electron configurations and a wider range of chemical and physical properties. The higher shells, however, become increasingly less effective at shielding the outer electrons from the nuclear charge, leading to more intricate interactions.
However, the fundamental principles we've discussed regarding electron capacity and subshells remain applicable. Each shell has a specific maximum capacity, determined by 2n², and the subshells (s, p, d, f) within each shell dictate the specific arrangement of electrons.
Conclusion: Understanding the Third Shell and Beyond
The third electron shell, with its capacity of 18 electrons distributed across 3s, 3p, and 3d subshells, plays a crucial role in determining the chemical and physical properties of many elements. Understanding its electron capacity, in conjunction with the principles of electron configuration and quantum mechanics, is essential for comprehending atomic structure and the periodic behavior of elements. This knowledge forms the bedrock for understanding chemical bonding, reactivity, and the properties of materials. This detailed exploration should provide a strong foundation for further investigation into the fascinating world of atomic structure and chemical behavior. Remember that while the 2n² rule offers a convenient guideline for maximum electron capacity, the actual filling order might exhibit slight variations due to the intricate energy level interplay within and between shells and subshells. The quantum mechanical description offers the most precise picture.
Latest Posts
Latest Posts
-
What Is 3 Divided By 11
Apr 02, 2025
-
How To Find The Mass Of The Excess Reactant
Apr 02, 2025
-
Instantaneous Rate Of Change Vs Average Rate Of Change
Apr 02, 2025
-
How Many D Orbitals Can Be In An Energy Level
Apr 02, 2025
-
Log Base 2 X 2 Graph
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The 3rd Shell Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
