How Many Electrons Can F Orbital Hold
listenit
Mar 24, 2025 · 5 min read
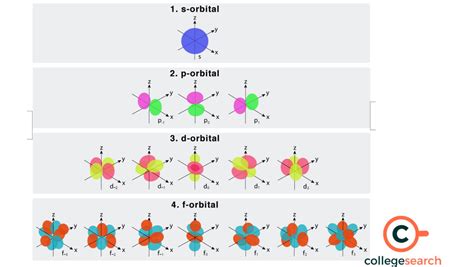
Table of Contents
How Many Electrons Can an F Orbital Hold? A Deep Dive into Atomic Structure
Understanding the electron capacity of atomic orbitals is fundamental to grasping the principles of chemistry. While s and p orbitals are relatively straightforward, the f orbital presents a slightly more complex scenario. This article delves deep into the question: how many electrons can an f orbital hold? We'll explore the quantum numbers that define orbitals, the Aufbau principle, Hund's rule, and the implications of f-orbital electron configuration on the properties of elements.
Understanding Atomic Orbitals and Quantum Numbers
Before we answer the central question, let's establish a solid foundation. Electrons within an atom occupy specific regions of space called atomic orbitals. These orbitals are defined by a set of four quantum numbers:
-
Principal Quantum Number (n): This number determines the energy level of the electron and the size of the orbital. It can take on positive integer values (n = 1, 2, 3,...). Higher n values correspond to higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): This describes the shape of the orbital and its angular momentum. It can have integer values ranging from 0 to n-1. The different values of l correspond to different subshells:
- l = 0: s orbital (spherical)
- l = 1: p orbital (dumbbell-shaped)
- l = 2: d orbital (more complex shapes)
- l = 3: f orbital (even more complex shapes)
-
Magnetic Quantum Number (ml): This specifies the orientation of the orbital in space. It can take on integer values from -l to +l, including 0. For example, an f orbital (l=3) has 7 possible orientations (ml = -3, -2, -1, 0, 1, 2, 3).
-
Spin Quantum Number (ms): This describes the intrinsic angular momentum of the electron, often referred to as its "spin." It can have only two values: +1/2 (spin up) or -1/2 (spin down).
The F Orbital: Shape and Degeneracy
The f orbital, characterized by l=3, is significantly more complex in shape than s, p, or d orbitals. It possesses seven different orientations in space, corresponding to the seven possible values of ml. These complex shapes are often visualized using intricate three-dimensional representations, but their precise forms aren't crucial for understanding electron capacity.
The key takeaway here is the degeneracy of the f orbital. Degeneracy refers to the fact that all seven f orbitals within a given energy level (n) have the same energy. This degeneracy is lifted (the orbitals have slightly different energies) in the presence of external fields or in molecules, but in isolated atoms, they remain degenerate.
Electron Capacity of an F Orbital: The Pauli Exclusion Principle
The fundamental principle governing the maximum number of electrons an orbital can hold is the Pauli Exclusion Principle. This principle states that no two electrons in an atom can have the same set of four quantum numbers.
Since each f orbital has seven possible orientations (ml values), and each electron can have one of two spin states (ms = +1/2 or -1/2), each f orbital can hold a maximum of two electrons. Therefore, considering the seven degenerate f orbitals, the total electron capacity of the f subshell is 14 electrons.
Filling F Orbitals: Aufbau Principle and Hund's Rule
The Aufbau principle dictates that electrons fill orbitals in order of increasing energy. Generally, electrons will first fill lower energy levels before moving to higher ones. However, the precise order of filling can become complex for heavier elements, as orbital energies can sometimes overlap.
Hund's rule states that electrons will individually occupy each orbital within a subshell before pairing up. This means that when filling f orbitals, electrons will first occupy each of the seven orbitals singly before pairing up in any orbital. This minimizes electron-electron repulsion and leads to a more stable configuration.
Implications of F-Orbital Electron Configuration
The f-orbital electrons play a critical role in the properties of the lanthanides and actinides, the elements located in the sixth and seventh rows of the periodic table, also known as the inner transition elements. These elements are characterized by the filling of the 4f and 5f orbitals, respectively.
The unique electron configuration of these elements results in several key characteristics:
-
Similar Chemical Properties: Due to the shielding effect of the inner 4f and 5f electrons, the chemical properties of the lanthanides and actinides are relatively similar within their respective series. This makes their separation and purification challenging.
-
Paramagnetism: Many lanthanides and actinides exhibit paramagnetism, a magnetic property arising from unpaired electrons in their f orbitals.
-
Colored Compounds: The presence of f electrons gives rise to the characteristic colors observed in many lanthanide and actinide compounds, a result of electronic transitions within the f subshell.
-
Radioactivity: The actinides are all radioactive, a consequence of their large nuclei and the instability of their f-orbital electron configurations.
Beyond the Basics: Relativistic Effects in Heavy Elements
For the heaviest elements, relativistic effects become significant and alter the energy levels of electrons. These effects can influence the order of orbital filling and even the electron capacity of some orbitals to a minor extent. Although it doesn't change the fundamental capacity of the f orbital (14 electrons), it can subtly affect its behavior and influence the overall chemical properties of these superheavy elements.
Conclusion: 14 Electrons and the Significance of the F Subshell
In conclusion, an f orbital can hold a maximum of 14 electrons. This fundamental fact stems from the combination of the Pauli Exclusion Principle, which limits each orbital to a maximum of two electrons, and the existence of seven degenerate f orbitals. The filling of these orbitals influences the properties of the lanthanides and actinides significantly, demonstrating the vital role of electron configuration in determining atomic and molecular behavior. Understanding the f orbital and its electron capacity is crucial for a deeper comprehension of the periodic table and the behavior of matter at the atomic level. The complexities introduced by the f-block elements demonstrate that even seemingly basic concepts in atomic structure can have far-reaching consequences. The continued study and refinement of our understanding of atomic structure and electronic configurations are critical in pushing the boundaries of chemistry and related fields.
Latest Posts
Latest Posts
-
What Percent Of 12 5 Is 39
Mar 26, 2025
-
Empirical And Molecular Formula Of Ibuprofen
Mar 26, 2025
-
What Is The Molar Mass Of So2
Mar 26, 2025
-
Electrons In The Outermost Energy Level Are Called
Mar 26, 2025
-
Express The Integral As A Limit Of Riemann Sums
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can F Orbital Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
