How Many Electrons Are In A Carbon Atom
listenit
Mar 21, 2025 · 6 min read
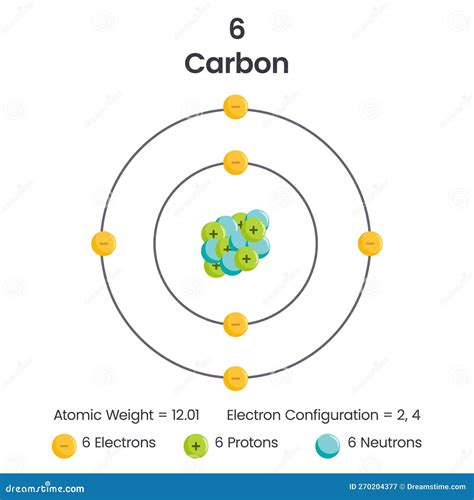
Table of Contents
How Many Electrons Are in a Carbon Atom? A Deep Dive into Atomic Structure
The seemingly simple question, "How many electrons are in a carbon atom?" opens a door to a fascinating exploration of atomic structure, chemical bonding, and the very foundation of chemistry. While the answer itself is straightforward, understanding why that number is significant requires delving into the intricacies of the atom. This article will not only answer the question but will also unpack the underlying concepts, exploring the role of electrons in chemical reactions and the unique properties of carbon that make it the building block of life.
The Straightforward Answer: Carbon's Electron Count
A neutral carbon atom contains six electrons. This is a fundamental piece of information derived from carbon's atomic number.
Understanding Atomic Number and Atomic Structure
The atomic number of an element is the defining characteristic that distinguishes it from all other elements. It represents the number of protons found in the nucleus of an atom. Since atoms are electrically neutral (unless they are ions), the number of protons always equals the number of electrons. Carbon's atomic number is 6; therefore, a neutral carbon atom possesses six protons and six electrons.
Protons, Neutrons, and Electrons: The Atomic Trio
Let's clarify the roles of these subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. They determine the element's identity.
- Neutrons: Neutral particles (no charge) also residing in the nucleus. They contribute to the atom's mass but not its charge. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. Their arrangement determines an atom's chemical behavior and reactivity.
Electron Shells and Orbitals: Organizing Electrons
Electrons don't simply zoom around the nucleus randomly. They occupy specific energy levels, often visualized as concentric shells surrounding the nucleus. Each shell can hold a limited number of electrons:
- Shell 1 (K-shell): Holds a maximum of 2 electrons.
- Shell 2 (L-shell): Holds a maximum of 8 electrons.
- Shell 3 (M-shell): Holds a maximum of 18 electrons (although for carbon, it's not relevant).
Within each shell are sub-shells (s, p, d, f), further subdivided into orbitals. Orbitals are regions of space where there is a high probability of finding an electron.
Carbon's Electron Configuration
For carbon, the six electrons are distributed as follows:
- Shell 1 (K-shell): 2 electrons (filling the 1s orbital)
- Shell 2 (L-shell): 4 electrons (filling the 2s orbital and two of the 2p orbitals)
This electron configuration is written as 1s²2s²2p². This specific arrangement is crucial to understanding carbon's exceptional bonding capabilities.
Carbon's Unique Bonding: The Key to its Versatility
Carbon's four valence electrons (the electrons in the outermost shell) are the key to its incredible versatility and ability to form a vast array of molecules. Valence electrons are responsible for chemical bonding, which involves the sharing or transfer of electrons between atoms.
Covalent Bonding: Sharing is Caring
Carbon primarily forms covalent bonds, sharing its four valence electrons with other atoms to achieve a stable electron configuration, typically resembling that of a noble gas (eight electrons in the outermost shell – the octet rule). This allows for the formation of long chains, branched structures, and rings – all crucial for the complexity of organic molecules.
Examples of Carbon's Bonding Prowess
The versatility of carbon's bonding is evident in the countless molecules it forms:
- Methane (CH₄): Carbon shares its four electrons with four hydrogen atoms, forming four strong single bonds.
- Ethane (C₂H₆): Two carbon atoms share one pair of electrons, forming a single bond, with the remaining electrons bonding with hydrogen atoms.
- Ethylene (C₂H₄): Two carbon atoms share two pairs of electrons, forming a double bond, and the remaining electrons bond with hydrogen.
- Acetylene (C₂H₂): Two carbon atoms share three pairs of electrons, forming a triple bond, with the remaining electrons bonding with hydrogen.
- Benzene (C₆H₆): A ring structure with alternating single and double bonds between carbon atoms, creating a delocalized electron system.
These examples represent just a tiny fraction of the vast array of organic molecules that rely on carbon's bonding capabilities.
Carbon's Role in Life: The Building Block of Life
Carbon's ability to form strong and stable covalent bonds with itself and other elements, particularly hydrogen, oxygen, nitrogen, and sulfur, is the basis for the incredible diversity of organic molecules found in living organisms. These molecules include:
- Carbohydrates: Sugars and starches, providing energy and structural support.
- Lipids: Fats and oils, storing energy and forming cell membranes.
- Proteins: Complex molecules responsible for a myriad of functions, including enzymes, structural components, and hormones.
- Nucleic acids: DNA and RNA, carrying genetic information.
The complexity and diversity of life are fundamentally rooted in carbon's unique chemistry.
Isotopes of Carbon: Variations in Neutron Count
While the number of electrons (and protons) defines the element, the number of neutrons can vary. These variations are called isotopes. The most common isotopes of carbon are:
- Carbon-12 (¹²C): 6 protons, 6 neutrons, 6 electrons. This is the most abundant isotope.
- Carbon-13 (¹³C): 6 protons, 7 neutrons, 6 electrons. A stable isotope used in various scientific applications.
- Carbon-14 (¹⁴C): 6 protons, 8 neutrons, 6 electrons. A radioactive isotope used in radiocarbon dating.
The number of electrons remains the same in all isotopes of carbon (6), because the number of electrons must balance the number of protons to ensure electrical neutrality. The difference in neutron number only affects the atomic mass.
Ions of Carbon: When Electrons are Lost or Gained
While a neutral carbon atom has six electrons, it can gain or lose electrons to form ions. This usually occurs in exceptional chemical environments. For instance, carbon can form a carbide ion (C⁴⁻) under certain conditions, though this is less common than its neutral or covalently bonded forms. This is a less stable configuration than the neutral atom or covalently bonded molecules. The creation of a carbon ion involves significant energy changes.
Conclusion: The Significance of Six Electrons
The seemingly simple answer – six electrons – to the question of how many electrons are in a carbon atom, unlocks a profound understanding of this element's importance in chemistry and biology. Its unique electron configuration, specifically the four valence electrons, allows for versatile bonding, ultimately enabling the incredible complexity and diversity of organic molecules that form the basis of life on Earth. Understanding the number of electrons is the first step in appreciating the profound role of carbon in our world.
Latest Posts
Latest Posts
-
What Is The Conjugate Acid Of Hso4
Mar 28, 2025
-
What Is The Name Of Fecl3
Mar 28, 2025
-
Whats Between 1 4 And 3 8
Mar 28, 2025
-
How To Find Radius Of A Circle Given Circumference
Mar 28, 2025
-
What Is The Average Atomic Mass Of Carbon
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In A Carbon Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
