How Many Electron Shells Does Carbon Have
listenit
Mar 29, 2025 · 6 min read
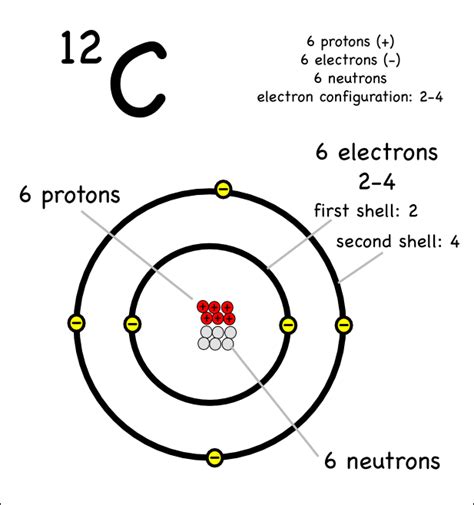
Table of Contents
How Many Electron Shells Does Carbon Have? A Deep Dive into Atomic Structure
Carbon, the cornerstone of organic chemistry and life itself, is a fascinating element with a surprisingly simple yet profoundly impactful atomic structure. Understanding its electron configuration is key to grasping its remarkable properties and its central role in the universe. So, how many electron shells does carbon have? The answer, while seemingly straightforward, opens the door to a deeper understanding of atomic theory, chemical bonding, and the periodic table.
The Basics: Unveiling Carbon's Atomic Structure
Carbon (C), with an atomic number of 6, possesses six protons in its nucleus. To maintain electrical neutrality, it also has six electrons orbiting this nucleus. These electrons are arranged in specific energy levels or shells, dictated by the principles of quantum mechanics. These shells are not simply concentric circles; they represent regions of space where there's a high probability of finding an electron.
Electron Shells and Subshells: A Closer Look
Electrons don't randomly occupy space around the nucleus. They are organized into shells, denoted by principal quantum numbers (n). Each shell can hold a maximum number of electrons, determined by the formula 2n², where 'n' is the shell number. The first shell (n=1) can hold a maximum of 2 electrons, the second shell (n=2) can hold up to 8 electrons, the third shell (n=3) can hold up to 18 electrons, and so on.
Within each shell, electrons are further categorized into subshells, designated by letters (s, p, d, f). These subshells represent different orbital shapes and energy levels. The 's' subshell has a spherical shape and can hold a maximum of 2 electrons. The 'p' subshell has a dumbbell shape and can hold up to 6 electrons. The 'd' and 'f' subshells have more complex shapes and can accommodate even more electrons.
Carbon's Electron Configuration: The Answer
Now, let's get to the heart of the matter: carbon has two electron shells.
- The first shell (n=1): This shell contains two electrons, completely filling the 1s subshell. This is a very stable configuration.
- The second shell (n=2): This shell contains the remaining four electrons. Two of these electrons fill the 2s subshell, while the other two occupy two of the three available orbitals in the 2p subshell. This leaves one 2p orbital empty.
This configuration is often represented as 1s²2s²2p². This notation tells us the number of electrons in each subshell. The superscript indicates the number of electrons in that particular subshell.
This seemingly simple arrangement is the key to carbon's extraordinary versatility and ability to form a vast array of molecules. The incomplete outer shell (2p) allows carbon to readily form covalent bonds with other atoms, sharing electrons to achieve a stable octet (eight electrons in its outermost shell).
The Significance of Carbon's Electron Shells
The presence of only two electron shells and the specific arrangement of electrons in those shells is the foundation of carbon's unique chemical behavior:
Covalent Bonding: The Cornerstone of Organic Chemistry
The four electrons in carbon's outer shell are readily available for sharing with other atoms, forming strong covalent bonds. This ability is crucial for the formation of long chains, branched structures, and ring structures—the building blocks of organic molecules. It's this capacity for diverse bonding that allows carbon to form the complex molecules essential for life.
Tetrahedral Geometry: A Consequence of Electron Arrangement
The arrangement of the four valence electrons in carbon (two in the 2s subshell and two in the 2p subshell) leads to a tetrahedral geometry. This means that the four bonds formed by carbon are arranged in a three-dimensional tetrahedron, maximizing the distance between them and minimizing repulsion. This geometry influences the shapes of organic molecules and their reactivity.
Hybridization: Tailoring Bonds for Specific Needs
Carbon’s ability to hybridize its orbitals is another significant consequence of its electron shell structure. Hybridization is the mixing of atomic orbitals to form new hybrid orbitals that have different shapes and energies. In carbon, the 2s and 2p orbitals can hybridize to form sp, sp², or sp³ hybrid orbitals, leading to different bond angles and molecular geometries. This flexibility in bonding is essential for the diversity of organic compounds.
Carbon's Role in Life: From Simple to Complex
The unique bonding capabilities of carbon, stemming directly from its two electron shells and electron configuration, are the basis for the incredible diversity of organic molecules found in living organisms. From simple sugars and amino acids to complex proteins and nucleic acids, carbon provides the structural framework and functional groups that allow for the complexity of life.
Comparing Carbon to Other Elements
Comparing carbon's electron shell structure with other elements helps highlight its unique properties. For instance:
- Hydrogen (H): Only one electron shell, with one electron in the 1s orbital. It forms only one bond.
- Oxygen (O): Two electron shells; the outer shell has six electrons. It typically forms two bonds.
- Nitrogen (N): Two electron shells; the outer shell has five electrons. It typically forms three bonds.
Carbon's four valence electrons allow it to form a greater number and variety of bonds than these other elements, leading to a vastly greater diversity of compounds.
Advanced Concepts: Relating Electron Shells to Periodic Trends
Understanding carbon's electron shell structure also provides insights into periodic trends:
- Ionization Energy: The energy required to remove an electron from an atom. Carbon's ionization energy reflects the stability of its electron configuration.
- Electronegativity: The ability of an atom to attract electrons in a chemical bond. Carbon's electronegativity is intermediate, allowing it to form bonds with a wide range of elements.
- Atomic Radius: The size of an atom. Carbon's atomic radius is relatively small, influencing its bonding properties.
These properties are all interconnected and are directly influenced by the number and arrangement of electrons in carbon's two electron shells.
Conclusion: The Profound Impact of a Simple Structure
The seemingly simple answer – carbon has two electron shells – underpins a vast array of chemical phenomena and biological processes. Its unique electron configuration leads to its remarkable versatility in forming covalent bonds, resulting in the unparalleled diversity of organic molecules crucial for life on Earth. By understanding the structure of carbon's electron shells, we gain a deeper appreciation for the fundamental principles of chemistry and the incredible complexity that can arise from a relatively simple atomic arrangement. This knowledge is essential not only for understanding the natural world but also for advancing fields like materials science, nanotechnology, and medicine.
Latest Posts
Latest Posts
-
What Is 8 25 As A Decimal
Mar 31, 2025
-
Which Type Of Chemical Bond Involves The Exchange Of Electrons
Mar 31, 2025
-
What Is The Charge For Chlorine
Mar 31, 2025
-
Finding Mole Ratio Practice Questions 1 Answer Key
Mar 31, 2025
-
How Many Atoms Are In Nitrogen
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Electron Shells Does Carbon Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
