How Is An Ion Different From An Atom
listenit
Mar 24, 2025 · 5 min read
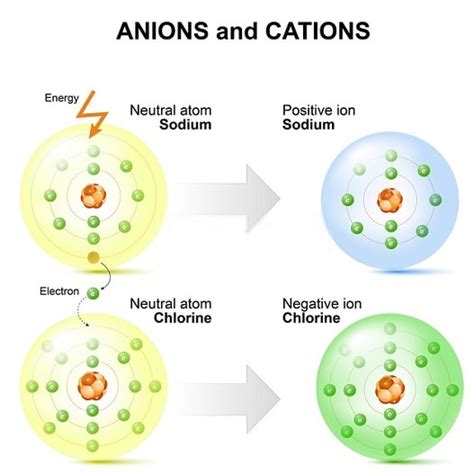
Table of Contents
How is an Ion Different from an Atom? Understanding the Fundamentals of Charge
Atoms and ions are fundamental building blocks of matter, but they differ significantly in one crucial aspect: electric charge. While atoms are generally neutral, ions carry a net electrical charge. This seemingly small difference has profound implications for their chemical behavior, physical properties, and roles in various natural phenomena. Understanding this distinction is key to grasping the complexities of chemistry and physics.
The Atom: A Neutral Entity
An atom, at its core, is defined by its composition of protons, neutrons, and electrons. Protons, carrying a positive charge (+1), reside in the atom's nucleus, along with neutrons, which are electrically neutral. Orbiting the nucleus are electrons, each possessing a negative charge (-1). In a neutral atom, the number of protons precisely equals the number of electrons, resulting in a net charge of zero. This balance of positive and negative charges is what defines the atom's overall neutrality.
Key Atomic Characteristics:
- Atomic Number: This determines the identity of an element and represents the number of protons in the nucleus. For instance, hydrogen has an atomic number of 1 (one proton), oxygen has 8, and gold has 79.
- Mass Number: This represents the total number of protons and neutrons in the nucleus. Isotopes of the same element have the same atomic number but different mass numbers due to variations in the number of neutrons.
- Electron Configuration: The arrangement of electrons in various energy levels (shells) around the nucleus dictates an atom's chemical reactivity. Atoms strive for a stable electron configuration, often by gaining, losing, or sharing electrons with other atoms.
The Ion: A Charged Particle
An ion, unlike a neutral atom, possesses a net electrical charge. This charge arises from an imbalance in the number of protons and electrons. Ions are formed when an atom either gains or loses electrons.
Cation: The Positively Charged Ion
A cation is a positively charged ion, formed when an atom loses one or more electrons. The loss of negatively charged electrons leaves the atom with more protons than electrons, resulting in a net positive charge. The magnitude of the positive charge is equal to the number of electrons lost. For example, a sodium atom (Na) readily loses one electron to become a sodium cation (Na⁺).
Examples of Cations:
- Na⁺ (Sodium ion): A common cation found in table salt (NaCl).
- Ca²⁺ (Calcium ion): Essential for strong bones and teeth.
- Fe²⁺ (Iron(II) ion) and Fe³⁺ (Iron(III) ion): Play vital roles in oxygen transport in blood.
- H⁺ (Hydrogen ion or proton): A crucial component in acid-base chemistry.
Anion: The Negatively Charged Ion
An anion is a negatively charged ion, formed when an atom gains one or more electrons. The addition of negatively charged electrons results in more electrons than protons, leading to a net negative charge. The magnitude of the negative charge is equal to the number of electrons gained. For example, a chlorine atom (Cl) readily gains one electron to become a chloride anion (Cl⁻).
Examples of Anions:
- Cl⁻ (Chloride ion): Found in table salt (NaCl) and stomach acid.
- O²⁻ (Oxide ion): A component of many metal oxides.
- SO₄²⁻ (Sulfate ion): Present in many salts and acids.
- PO₄³⁻ (Phosphate ion): Essential for energy storage and transfer in biological systems.
The Formation of Ions: Ionization
The process of forming ions is known as ionization. This process can occur through various mechanisms, including:
- Electron Transfer: This is the most common method, involving the transfer of electrons from one atom to another. This often happens between atoms with significantly different electronegativities (the tendency of an atom to attract electrons).
- Electromagnetic Radiation: High-energy electromagnetic radiation, such as X-rays or gamma rays, can ionize atoms by stripping away electrons.
- Collision with Particles: Collisions with high-energy particles, such as those found in radioactive decay or in particle accelerators, can also knock electrons out of atoms.
The Importance of Ions
Ions play crucial roles in numerous natural processes and technological applications:
Biological Systems:
- Nerve Impulse Transmission: The movement of ions (sodium, potassium, calcium) across cell membranes is essential for nerve impulse transmission.
- Muscle Contraction: Similar ionic movements trigger muscle contractions.
- Enzyme Function: Many enzymes require specific ions for their catalytic activity.
- Osmosis and Water Balance: Ion concentrations play a crucial role in maintaining osmotic balance and fluid regulation within cells and organisms.
Chemical Reactions:
- Ionic Compounds: Ions are the building blocks of ionic compounds, such as salts and oxides. These compounds are formed through electrostatic attraction between oppositely charged ions.
- Electrolytes: Ions in solution conduct electricity, making them essential components of electrolytes. These are vital in various industrial processes and energy storage systems.
Industrial Applications:
- Electroplating: Electroplating uses ions to deposit a thin layer of metal onto a surface.
- Batteries: Batteries rely on the movement of ions to generate electrical current.
- Corrosion: Corrosion is often driven by the movement and reaction of ions.
Distinguishing Features: Atoms vs. Ions
The following table summarizes the key differences between atoms and ions:
| Feature | Atom | Ion |
|---|---|---|
| Charge | Neutral (no net charge) | Possesses a net electric charge |
| Electron Number | Equal to proton number | Unequal to proton number |
| Formation | Fundamental building block of matter | Formed through ionization of an atom |
| Chemical Reactivity | Varies depending on electron configuration | High, driven by electrostatic forces |
| Examples | H, O, Na, Cl | H⁺, O²⁻, Na⁺, Cl⁻ |
Conclusion: A Fundamental Distinction with Profound Implications
The difference between an atom and an ion, while seemingly subtle – the presence or absence of a net charge – has profound consequences for the behavior and properties of matter. Atoms, the neutral building blocks, form the foundation of all elements. Ions, their charged counterparts, drive crucial biological processes, chemical reactions, and numerous industrial applications. Understanding this fundamental distinction is key to comprehending the intricacies of the physical and chemical world around us, unlocking a deeper understanding of matter's behavior at the atomic level and beyond. From the simple elegance of salt crystals to the complex mechanisms of life itself, ions play an undeniable and vital role.
Latest Posts
Latest Posts
-
What Is The Molar Mass Of So2
Mar 26, 2025
-
Electrons In The Outermost Energy Level Are Called
Mar 26, 2025
-
Express The Integral As A Limit Of Riemann Sums
Mar 26, 2025
-
How Many Lines Of Symmetry Rectangle
Mar 26, 2025
-
How Many Feet Are In 15 Miles
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Is An Ion Different From An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
