How Do You Calculate Freezing Point Depression
listenit
Mar 18, 2025 · 6 min read
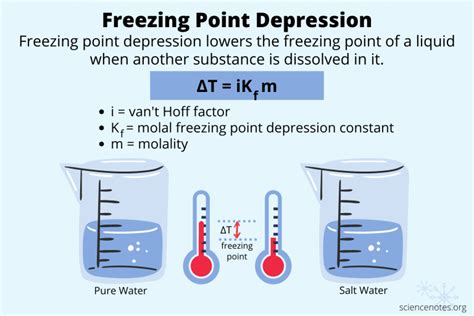
Table of Contents
How Do You Calculate Freezing Point Depression? A Comprehensive Guide
Freezing point depression is a colligative property, meaning it depends on the number of solute particles dissolved in a solvent, not their identity. This phenomenon is crucial in various applications, from de-icing roads to understanding biological processes. This comprehensive guide will delve into the intricacies of calculating freezing point depression, covering the fundamental principles, the necessary equations, and examples to solidify your understanding.
Understanding the Fundamentals
Before diving into the calculations, let's grasp the underlying principles. When a solute dissolves in a solvent, it disrupts the solvent's crystal lattice structure, making it more difficult for the solvent molecules to arrange themselves into a solid state. This increased difficulty requires a lower temperature to achieve solidification, hence the depression of the freezing point.
Key Concepts:
- Solvent: The substance doing the dissolving (e.g., water).
- Solute: The substance being dissolved (e.g., salt, sugar).
- Freezing Point Depression (ΔTf): The difference between the freezing point of the pure solvent and the freezing point of the solution.
- Molality (m): The concentration of the solute expressed as moles of solute per kilogram of solvent. This is crucial because colligative properties depend on the ratio of solute particles to solvent molecules, and molality provides a consistent measure of this ratio regardless of the volume of the solution.
The Equation: Unveiling the Calculation
The fundamental equation for calculating freezing point depression is:
ΔTf = Kf * m * i
Where:
- ΔTf represents the freezing point depression (in °C or K).
- Kf is the cryoscopic constant of the solvent (a unique property for each solvent, expressed in °C kg/mol or K kg/mol). This constant reflects how susceptible the solvent is to freezing point depression. Water, for instance, has a Kf of 1.86 °C kg/mol.
- m is the molality of the solution (mol/kg).
- i is the van't Hoff factor, representing the number of particles the solute dissociates into when dissolved. This is crucial for ionic compounds.
Let's break down each component in more detail:
1. Cryoscopic Constant (Kf): A Solvent-Specific Property
The cryoscopic constant (Kf) is a characteristic property of the solvent. It indicates the extent to which the freezing point of the solvent will be lowered by the addition of one mole of solute particles per kilogram of solvent. Different solvents have different Kf values. For instance:
- Water: Kf = 1.86 °C kg/mol
- Benzene: Kf = 5.12 °C kg/mol
- Cyclohexane: Kf = 20.0 °C kg/mol
- Camphor: Kf = 37.7 °C kg/mol
The higher the Kf value, the greater the freezing point depression for a given molality of solute.
2. Molality (m): Concentration Matters
Molality (m) is the key concentration unit in freezing point depression calculations. It's defined as the number of moles of solute per kilogram of solvent. The formula for molality is:
m = (moles of solute) / (kilograms of solvent)
To calculate molality, you need to know the number of moles of solute and the mass of the solvent in kilograms. Remember, it is crucial to use kilograms of solvent, not the total mass of the solution.
3. Van't Hoff Factor (i): Accounting for Dissociation
The van't Hoff factor (i) accounts for the dissociation of ionic compounds in solution. For non-electrolytes (substances that do not dissociate into ions), i = 1. However, for ionic compounds, i is greater than 1 because they dissociate into multiple ions. For example:
- NaCl (sodium chloride): i ≈ 2 (dissociates into Na⁺ and Cl⁻)
- MgCl₂ (magnesium chloride): i ≈ 3 (dissociates into Mg²⁺ and 2Cl⁻)
- Glucose (a non-electrolyte): i = 1
In reality, the van't Hoff factor is often less than the theoretically predicted value due to ion pairing, where ions are attracted to each other and do not behave as completely independent particles. The actual value of 'i' might need to be determined experimentally, or approximated based on the concentration and nature of the solution.
Step-by-Step Calculation: Putting it All Together
Let's work through a detailed example to solidify your understanding.
Problem: Calculate the freezing point depression of a solution containing 10 grams of NaCl dissolved in 100 grams of water.
Step 1: Calculate the moles of NaCl:
- The molar mass of NaCl is approximately 58.44 g/mol.
- Moles of NaCl = (10 g) / (58.44 g/mol) = 0.171 moles
Step 2: Calculate the molality (m):
- Mass of water in kilograms = 100 g / 1000 g/kg = 0.1 kg
- Molality (m) = (0.171 moles) / (0.1 kg) = 1.71 mol/kg
Step 3: Determine the van't Hoff factor (i):
- NaCl dissociates into two ions (Na⁺ and Cl⁻), so i ≈ 2. (Remember, this is an approximation; the actual value might be slightly less due to ion pairing).
Step 4: Calculate the freezing point depression (ΔTf):
- Kf for water = 1.86 °C kg/mol
- ΔTf = Kf * m * i = (1.86 °C kg/mol) * (1.71 mol/kg) * (2) = 6.36 °C
Step 5: Calculate the new freezing point:
- The freezing point of pure water is 0 °C.
- New freezing point = 0 °C - 6.36 °C = -6.36 °C
Therefore, the freezing point of the solution is approximately -6.36 °C.
Advanced Considerations and Applications
While the basic equation provides a good approximation, several factors can influence the accuracy of the calculation:
- Ion Pairing: As mentioned, ion pairing can reduce the effective van't Hoff factor, especially at higher concentrations.
- Non-ideality: At higher concentrations, the solution may deviate from ideal behavior, affecting the accuracy of the calculations. Activity coefficients can be introduced to correct for this deviation, though this adds complexity.
- Association of molecules: Some solutes can associate in solution, forming dimers or larger aggregates, leading to a lower effective concentration of particles and thus a smaller freezing point depression.
- Solubility limitations: The calculations are only valid if the solute completely dissolves in the solvent.
Real-world Applications: Beyond the Textbook
Freezing point depression has numerous practical applications:
- De-icing: Spreading salt on icy roads lowers the freezing point of water, preventing ice formation at temperatures below 0°C.
- Antifreeze: Ethylene glycol is added to car radiators to lower the freezing point of water, preventing damage to the engine during winter.
- Food preservation: Adding salt or sugar to food lowers the freezing point, helping to preserve food by preventing the formation of ice crystals that can damage cell structures.
- Determining molar mass: Freezing point depression can be used to experimentally determine the molar mass of an unknown solute. By measuring the freezing point depression of a solution with a known mass of solute, the molality can be calculated, and hence the molar mass can be derived.
- Cryobiology: Understanding freezing point depression is crucial in cryobiology, the study of the effects of low temperatures on living organisms. Controlled freezing and thawing procedures are developed based on the knowledge of freezing point depression to minimize damage to cells and tissues.
Conclusion: Mastering Freezing Point Depression
Understanding and calculating freezing point depression is essential for anyone working with solutions. This guide has provided a comprehensive overview of the underlying principles, the necessary equations, and the practical applications of this crucial colligative property. While the basic equation provides a good approximation, it's important to consider the factors that can affect the accuracy of the calculation, especially in more complex scenarios. By mastering this concept, you gain a valuable tool for tackling problems in chemistry, biology, and various engineering disciplines.
Latest Posts
Latest Posts
-
How Many Millimeters Are In 6 Centimeters
Mar 18, 2025
-
Whats The Square Root Of 34
Mar 18, 2025
-
What Is 1 21 Repeating As A Fraction
Mar 18, 2025
-
What Particles Orbit Around The Nucleus
Mar 18, 2025
-
Water Boiling Is A Physical Change
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Do You Calculate Freezing Point Depression . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
