Water Boiling Is A Physical Change
listenit
Mar 18, 2025 · 5 min read
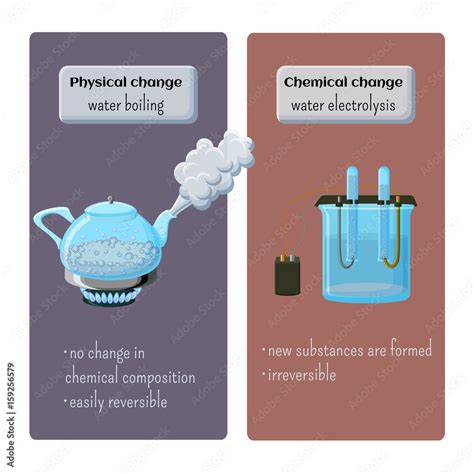
Table of Contents
- Water Boiling Is A Physical Change
- Table of Contents
- Water Boiling: A Deep Dive into a Physical Change
- Understanding Physical vs. Chemical Changes
- The Science Behind Boiling Water
- Kinetic Energy and Molecular Movement
- The Role of Heat
- Reaching the Boiling Point
- The Transition to Gas
- Condensation: The Reverse Process
- Evidence Supporting Boiling as a Physical Change
- Common Misconceptions about Boiling Water
- Boiling Water in Different Contexts
- Conclusion: Boiling Water – A Physical Transformation
- Latest Posts
- Latest Posts
- Related Post
Water Boiling: A Deep Dive into a Physical Change
Water boiling is a ubiquitous phenomenon, a daily occurrence for most people around the globe. But what exactly is happening when water boils? Is it a chemical change, transforming the water into something fundamentally different? Or is it a physical change, altering the water's state but not its inherent composition? The answer, unequivocally, is that boiling water is a physical change. This article will delve into the scientific intricacies of this process, dispelling any misconceptions and solidifying your understanding of this fundamental concept.
Understanding Physical vs. Chemical Changes
Before diving into the specifics of boiling water, it's crucial to establish a clear understanding of the difference between physical and chemical changes.
-
Physical Change: A physical change alters the form or appearance of a substance but doesn't change its chemical composition. Think of cutting paper, melting ice, or dissolving sugar in water. These processes change the state or form of the substance, but the molecules themselves remain unchanged. The substance can often be returned to its original state.
-
Chemical Change: A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules, resulting in the formation of new substances with different properties. Examples include burning wood, rusting iron, or baking a cake. These processes create entirely new substances with different chemical formulas. The original substances are usually not recoverable.
The Science Behind Boiling Water
Water (H₂O) exists in three main states: solid (ice), liquid (water), and gas (water vapor or steam). The boiling process is a transition from the liquid state to the gaseous state. This transition is driven by the kinetic energy of the water molecules.
Kinetic Energy and Molecular Movement
At the molecular level, water molecules are constantly in motion, vibrating and colliding with each other. The amount of kinetic energy these molecules possess is directly related to temperature. As temperature increases, so does the kinetic energy.
The Role of Heat
When heat is applied to liquid water, the molecules absorb this energy, increasing their kinetic energy. This increased kinetic energy leads to more vigorous movement and collisions.
Reaching the Boiling Point
As the water continues to heat, a specific temperature is reached where the kinetic energy of the water molecules overcomes the attractive forces holding them together in the liquid state. This temperature is the boiling point, which is 100°C (212°F) at standard atmospheric pressure.
The Transition to Gas
At the boiling point, the water molecules gain enough energy to break free from the liquid's surface and escape into the atmosphere as water vapor or steam. This is the process of vaporization or boiling. Noticeably, bubbles of steam form within the liquid itself as the water vapor expands.
Condensation: The Reverse Process
Importantly, the reverse process – condensation – is also a physical change. When water vapor cools down, it loses kinetic energy, and the attractive forces between the water molecules become dominant again. This causes the water vapor to return to its liquid state. This reversibility further reinforces the fact that boiling is a physical change.
Evidence Supporting Boiling as a Physical Change
Several key observations solidify the classification of boiling as a physical change:
-
No new substance is formed: Boiling water remains chemically H₂O, even after transitioning to steam. No new chemical bonds are formed or broken during the process. The chemical formula of water remains unchanged.
-
The process is reversible: Condensation, the reverse process, readily converts the steam back into liquid water. This reversibility is a hallmark of physical changes.
-
No change in chemical properties: The chemical properties of water – its ability to dissolve certain substances, its pH, its reactivity with other chemicals – remain constant throughout the boiling process.
-
Separation by physical means: Boiling is a method of separation based on differences in boiling points, which is a physical property. Distillation, a technique widely used in chemistry and industry, relies on this principle to separate mixtures of liquids based on their differing boiling points.
Common Misconceptions about Boiling Water
Despite the overwhelming scientific evidence, several misconceptions surround boiling water:
-
Boiling changes the taste of water: While some minerals might precipitate out of solution during boiling, altering the mineral content slightly, this doesn’t represent a chemical change in the water molecule itself. The change in taste is due to the removal or concentration of dissolved solids, not a transformation of H₂O.
-
Boiling water makes it sterile: While boiling effectively kills many harmful microorganisms, this is a physical process of denaturation— the disruption of the proteins in microorganisms—not a chemical alteration of the water. The chemical composition of the water remains largely the same.
-
Boiling water "purifies" it: Boiling can remove some dissolved gases and volatile organic compounds, but it doesn't remove dissolved minerals or heavy metals. For true purification, more advanced methods like filtration or reverse osmosis are necessary.
Boiling Water in Different Contexts
The principles of boiling water apply across various situations and contexts:
-
Cooking: Boiling is a fundamental method used in cooking to soften food, sterilize utensils, and prepare various dishes.
-
Industrial Processes: Boiling is used in numerous industrial processes, including distillation, sterilization, and the generation of steam for power production.
-
Scientific Experiments: Boiling is an essential technique in various scientific experiments, particularly those involving separating components of a mixture or studying phase transitions.
-
Weather Patterns: The evaporation of water from oceans, lakes, and rivers, followed by condensation in the atmosphere, plays a crucial role in weather patterns and the water cycle.
Conclusion: Boiling Water – A Physical Transformation
In conclusion, the boiling of water is unequivocally a physical change. It involves a transition between the liquid and gaseous states of water, driven by changes in the kinetic energy of water molecules. No new substances are formed, the process is reversible, and the chemical properties of water remain unaltered. Understanding this fundamental distinction is crucial for comprehending a wide range of scientific principles, industrial processes, and everyday phenomena. While boiling may alter some properties of the water, such as the taste or mineral content, it is a change in its physical state, not its chemical identity, that lies at the heart of this common and crucial process. The reversibility, lack of formation of a new substance, and unchanged chemical composition conclusively demonstrate that boiling water is a purely physical transformation.
Latest Posts
Latest Posts
-
What Is The Atomic Number For Neon
Mar 19, 2025
-
Can Be Standard Deviation Be Calc If Not Independent
Mar 19, 2025
-
What Percent Of 90 Is 25
Mar 19, 2025
-
What Is The Molar Mass Of Ammonium Sulfate
Mar 19, 2025
-
1 Square Foot Is How Many Square Inches
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Water Boiling Is A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
