What Is The Atomic Number For Neon
listenit
Mar 19, 2025 · 6 min read
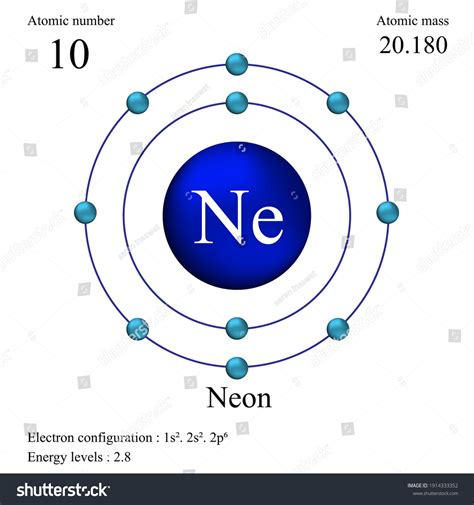
Table of Contents
What is the Atomic Number for Neon? A Deep Dive into Neon's Properties and Applications
Neon, the vibrant gas that illuminates our signs and plays a crucial role in various technologies, holds a fascinating place in the periodic table. Understanding its atomic number is key to unlocking its unique properties and appreciating its widespread applications. This comprehensive guide will delve deep into the world of neon, exploring its atomic structure, characteristics, uses, and its significance in science and technology.
Understanding Atomic Numbers: The Foundation of Chemistry
Before we reveal neon's atomic number, let's establish the fundamental concept of atomic number. The atomic number of an element represents the number of protons found in the nucleus of a single atom of that element. Protons, along with neutrons, constitute the nucleus, while electrons orbit the nucleus in shells or energy levels. The atomic number uniquely identifies an element and determines its chemical properties. It's the cornerstone of the periodic table, organizing elements based on their increasing atomic numbers and reflecting their recurring chemical behaviors.
Neon's Atomic Number: Unveiling the Mystery
The atomic number for neon is 10. This signifies that every neon atom contains 10 protons in its nucleus. This fundamental characteristic dictates neon's behavior and differentiates it from other elements. Because atoms are electrically neutral, a neon atom with 10 protons also possesses 10 electrons, balancing the positive charge of the protons. This electron configuration determines neon's chemical inertness, a property that contributes significantly to its diverse applications.
Neon's Electron Configuration: The Key to Inertness
Neon's 10 electrons are arranged in two energy levels: two electrons in the first energy level (the innermost shell) and eight electrons in the second energy level. This arrangement, often represented as 1s²2s²2p⁶, is exceptionally stable. The outermost shell, the valence shell, is completely filled with eight electrons, achieving what's known as an octet. This complete octet makes neon highly unreactive, or inert. It resists forming chemical bonds with other elements because it already possesses a stable electron configuration, making it a noble gas.
Properties of Neon: A Closer Look
Neon's atomic number and resulting electron configuration are directly responsible for its distinctive properties:
1. Inertness:
As mentioned earlier, neon's complete octet leads to its chemical inertness. It doesn't readily react with other elements, making it safe for various applications where reactivity would be undesirable.
2. Gaseous State:
At standard temperature and pressure, neon exists as a gas. Its weak interatomic forces, a consequence of its stable electron configuration, prevent the formation of strong bonds that would lead to a solid or liquid state under normal conditions.
3. Low Density:
Neon gas is significantly less dense than air. This low density contributes to its use in applications requiring a lightweight gas.
4. Colorless and Odorless:
In its pure form, neon gas is colorless and odorless, adding to its suitability for diverse applications where visual or olfactory neutrality is preferred.
5. Electrical Conductivity:
While inert chemically, neon exhibits electrical conductivity when subjected to high voltage. This property is central to its use in lighting applications. When an electric current passes through neon gas, it emits a characteristic reddish-orange glow, a phenomenon exploited in neon signs.
Applications of Neon: From Signs to Lasers
The unique properties stemming from neon's atomic number translate into a remarkable range of applications:
1. Neon Lighting:
This is perhaps the most recognizable application of neon. High-voltage electricity passing through neon gas contained in glass tubes causes the gas to emit a vibrant reddish-orange light. This principle underpins the iconic neon signs that illuminate cities worldwide. While the term "neon sign" is commonly used, many such signs actually use other gases to produce a wider spectrum of colors. However, neon itself provides that distinct and classic reddish-orange glow.
2. Lasers:
Neon is used in combination with other gases in helium-neon lasers. These lasers produce a coherent beam of red light, finding applications in various scientific instruments, barcode scanners, and laser pointers. The mixture of helium and neon optimizes the laser's efficiency and power output.
3. Cryogenics:
Liquid neon, produced by cooling gaseous neon to extremely low temperatures, is employed as a cryogenic refrigerant. It's used in various scientific and industrial applications that require very low temperatures, offering an alternative to more expensive liquid helium.
4. High-Voltage Indicators:
Neon's glow discharge properties make it suitable for inclusion in high-voltage indicators. These indicators visibly signal the presence of high voltage, serving as a crucial safety feature in electrical equipment.
5. Vacuum Tubes:
Neon was historically used in some types of vacuum tubes, contributing to their operation and stability. While modern technology has largely replaced these tubes, their historical significance remains.
Neon's Abundance and Extraction: A Look at the Source
Neon, while not as abundant as some other elements, is readily available in the Earth's atmosphere. It's extracted through a process called fractional distillation of liquid air. This involves cooling air to its liquid state and then carefully separating its constituent gases based on their differing boiling points. Neon, with its relatively low boiling point, is one of the gases separated during this process.
Neon's Isotopes: Variations on a Theme
While neon's atomic number is always 10 (meaning 10 protons), the number of neutrons in its nucleus can vary, resulting in different isotopes. The most common isotopes are neon-20, neon-21, and neon-22. These isotopes differ in their mass number (the sum of protons and neutrons) but share the same chemical properties due to the identical number of protons.
Neon in the Wider Scientific Context: Research and Discoveries
Understanding neon's atomic number and properties has played a crucial role in various scientific advancements. Its use in lasers has revolutionized spectroscopy and enabled numerous scientific breakthroughs. The study of neon's behavior under extreme conditions contributes to our understanding of matter and energy at the atomic level. Moreover, its application in cryogenics has facilitated research in low-temperature physics and materials science.
Conclusion: The Significance of Atomic Number 10
The atomic number for neon, 10, isn't just a number; it's the key to unlocking the unique properties and diverse applications of this fascinating element. Its stable electron configuration leads to its inertness, enabling its safe use in various technological and scientific applications. From illuminating our cities to powering lasers, neon's contribution to modern technology is undeniable. Understanding its atomic structure provides insight into the fundamental principles of chemistry and the powerful link between an element's atomic structure and its macroscopic behavior. The continued research and exploration of neon's properties promise further scientific advancements and technological innovations in the years to come.
Latest Posts
Latest Posts
-
What Is The Percent Of 17 20
Mar 19, 2025
-
What Is Square Root Of 130
Mar 19, 2025
-
Convert Rectangular Coordinates To Polar Coordinates
Mar 19, 2025
-
What Do Animal Cells And Plant Cells Have In Common
Mar 19, 2025
-
What Is The Least Common Multiple Of 8 And 7
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Atomic Number For Neon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
