Electron Transport Takes Place In The
listenit
Mar 24, 2025 · 7 min read
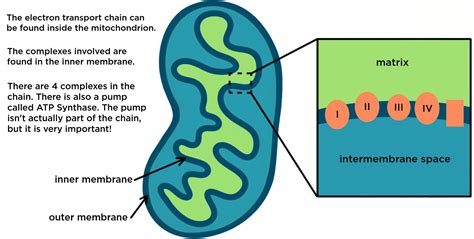
Table of Contents
Electron Transport Takes Place In The: A Deep Dive into Mitochondrial Respiration
Electron transport, a critical process in cellular respiration, is the powerhouse of life, responsible for generating the majority of the ATP (adenosine triphosphate) that fuels cellular activities. But where exactly does this vital process take place? The answer is primarily within the mitochondria, often referred to as the "powerhouses of the cell." This article will delve into the intricacies of electron transport, exploring its location within the mitochondria, the key players involved, the mechanisms driving ATP synthesis, and the broader implications for cellular function and human health.
The Mitochondrion: The Site of Cellular Respiration
The mitochondrion, a double-membrane-bound organelle, possesses a unique structure perfectly adapted for its role in energy production. Its two membranes – the outer mitochondrial membrane and the inner mitochondrial membrane – create distinct compartments crucial for electron transport.
The Outer Mitochondrial Membrane: A Gatekeeper
The outer mitochondrial membrane is relatively permeable, allowing the passage of small molecules. This permeability is facilitated by porins, protein channels that regulate the entry and exit of substances. While not directly involved in electron transport itself, the outer membrane plays an indirect role by regulating the flow of metabolites into the intermembrane space, the region between the outer and inner membranes.
The Intermembrane Space: A Critical Transition Zone
The intermembrane space, located between the outer and inner mitochondrial membranes, serves as a critical transition zone. Protons (H+) are pumped into this space during electron transport, creating a proton gradient that is essential for ATP synthesis. This gradient represents stored energy, driving the ATP synthase enzyme. The accumulation of protons in the intermembrane space lowers its pH, further contributing to the electrochemical gradient.
The Inner Mitochondrial Membrane: The Heart of Electron Transport
The inner mitochondrial membrane is the true site of electron transport. It is highly folded into cristae, significantly increasing its surface area. This increased surface area is crucial because it accommodates the numerous protein complexes involved in electron transport and ATP synthesis. The inner membrane is impermeable to most ions and molecules, ensuring that the proton gradient established during electron transport is maintained. This impermeability is critical for the efficient production of ATP.
Embedded within the inner mitochondrial membrane are four major protein complexes:
-
Complex I (NADH dehydrogenase): This complex accepts electrons from NADH, a high-energy electron carrier produced during glycolysis and the citric acid cycle. The transfer of electrons through Complex I pumps protons from the mitochondrial matrix into the intermembrane space.
-
Complex II (succinate dehydrogenase): Unlike Complex I, Complex II accepts electrons from FADH2, another high-energy electron carrier generated during the citric acid cycle. Complex II does not directly contribute to proton pumping, but it delivers electrons to the electron transport chain.
-
Complex III (cytochrome bc1 complex): Complex III receives electrons from Complex I or Complex II and passes them to cytochrome c, a mobile electron carrier. This transfer also involves proton pumping into the intermembrane space.
-
Complex IV (cytochrome c oxidase): The final electron acceptor in the chain, Complex IV receives electrons from cytochrome c. Oxygen (O2) acts as the terminal electron acceptor, reducing it to water (H2O). This step also involves proton pumping, contributing significantly to the proton gradient.
The Electron Transport Chain: A Cascade of Redox Reactions
The electron transport chain is a series of redox reactions (reduction-oxidation reactions) where electrons are passed from one molecule to another. Each electron carrier in the chain has a progressively higher electronegativity, meaning it has a stronger tendency to hold electrons. This sequential transfer of electrons releases energy, which is used to pump protons into the intermembrane space, establishing the proton motive force.
The Role of Electron Carriers
Several types of electron carriers participate in the electron transport chain, including:
-
Flavoproteins: These proteins contain flavin mononucleotide (FMN) or flavin adenine dinucleotide (FAD), which can accept and donate electrons.
-
Iron-sulfur proteins: These proteins contain iron-sulfur clusters that facilitate electron transfer.
-
Cytochromes: These proteins contain heme groups that can accept and donate electrons. Cytochromes are characterized by their absorbance of light at specific wavelengths.
Oxygen: The Final Electron Acceptor
Oxygen is crucial as the terminal electron acceptor in the electron transport chain. Without oxygen, the electron transport chain would become blocked, halting ATP production. This is why oxygen is essential for aerobic respiration.
ATP Synthase: Harnessing the Proton Gradient
The proton gradient established across the inner mitochondrial membrane provides the potential energy required for ATP synthesis. This energy is harnessed by ATP synthase, a remarkable molecular machine embedded in the inner membrane.
The Structure and Function of ATP Synthase
ATP synthase is a remarkable enzyme composed of two main components: F0 and F1. F0 forms a channel in the inner membrane allowing protons to flow back into the mitochondrial matrix down their electrochemical gradient. This proton flow drives the rotation of F0, which in turn drives the rotation of F1, the catalytic subunit. The rotation of F1 causes conformational changes that facilitate the synthesis of ATP from ADP and inorganic phosphate (Pi).
Chemiosmosis: Linking Proton Flow to ATP Synthesis
The process linking the proton gradient to ATP synthesis is known as chemiosmosis. The flow of protons back into the matrix through ATP synthase is coupled to the synthesis of ATP. The energy stored in the proton gradient is directly converted into the chemical energy stored in ATP molecules. This remarkable efficiency is essential for cellular energy metabolism.
Beyond ATP: Other Functions of the Electron Transport Chain
While ATP synthesis is the primary function of the electron transport chain, it also plays a crucial role in other cellular processes:
-
Reactive oxygen species (ROS) production: While mostly efficient, the electron transport chain can generate reactive oxygen species (ROS) as byproducts. While some ROS have cellular signaling functions, excessive ROS production can lead to oxidative stress, damaging cellular components and contributing to aging and diseases.
-
Regulation of cellular metabolism: The electron transport chain is tightly regulated to meet the changing energy demands of the cell. Various factors, including nutrient availability and oxygen levels, influence its activity.
-
Thermoregulation: In some organisms, such as brown adipose tissue in mammals, the electron transport chain generates heat instead of ATP, contributing to thermoregulation. This process is known as non-shivering thermogenesis.
Disruptions in Electron Transport: Implications for Human Health
Disruptions in the electron transport chain can have significant consequences for human health. Mutations in genes encoding components of the electron transport chain can lead to mitochondrial diseases, a group of disorders affecting various organs and tissues. These disorders can manifest with a wide range of symptoms depending on which components are affected and the extent of the dysfunction.
Moreover, malfunctions in the electron transport chain have been implicated in numerous other health issues, including:
-
Neurodegenerative diseases: Mitochondrial dysfunction plays a role in diseases like Alzheimer's and Parkinson's disease.
-
Cardiovascular disease: Impaired mitochondrial function contributes to heart failure and other cardiovascular problems.
-
Cancer: Mitochondrial dysfunction can influence cancer development, progression, and metastasis.
-
Diabetes: Impaired mitochondrial function contributes to insulin resistance and type 2 diabetes.
Understanding the intricate mechanisms of the electron transport chain and its critical role in cellular energy metabolism is crucial for developing therapeutic strategies for these diverse diseases.
Conclusion: The Powerhouse Within
The electron transport chain, located within the inner mitochondrial membrane, is the engine driving the majority of ATP production in eukaryotic cells. Its precise location, complex interplay of protein complexes, and efficient mechanism for harnessing the proton gradient to synthesize ATP highlight the elegance and efficiency of cellular processes. Understanding this fundamental process is crucial for appreciating the complexity of cellular life and its implications for human health. Continued research in this area holds immense promise for developing treatments for various diseases stemming from mitochondrial dysfunction, emphasizing the enduring importance of this critical cellular process.
Latest Posts
Latest Posts
-
How Many Drops Are In 1ml
Mar 26, 2025
-
What Is The Correct Formula For Barium Phosphate
Mar 26, 2025
-
Is 37 A Prime Or Composite Number
Mar 26, 2025
-
How Many Valence Electrons In P
Mar 26, 2025
-
What Causes The Alpha Particles To Deflect Backwards
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Electron Transport Takes Place In The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
