What Causes The Alpha Particles To Deflect Backwards
listenit
Mar 26, 2025 · 5 min read
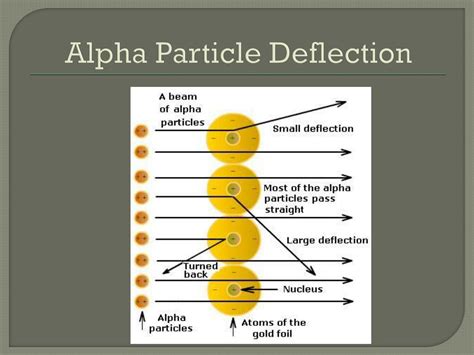
Table of Contents
What Causes Alpha Particles to Deflect Backwards? – Unveiling the Secrets of Rutherford's Gold Foil Experiment
The year is 1909. A groundbreaking experiment is underway at the University of Manchester, led by Ernest Rutherford. His team, including Hans Geiger and Ernest Marsden, is bombarding a thin gold foil with alpha particles – positively charged particles emitted by certain radioactive elements. The expectation? A straightforward passage of the alpha particles through the foil, with only minor deflections. The reality? A shocking discovery: a small percentage of alpha particles were deflected backwards, a phenomenon that completely overturned existing atomic models. This unexpected result led to the revolutionary nuclear model of the atom, forever changing our understanding of matter.
The Prevailing Atomic Model: The Plum Pudding
Before Rutherford's experiment, the accepted model of the atom was the "plum pudding" model proposed by J.J. Thomson. This model envisioned the atom as a sphere of positive charge with negatively charged electrons embedded within it, much like plums scattered in a pudding. This model predicted minimal deflection of alpha particles as the positive charge was assumed to be uniformly distributed, resulting in only minor electrostatic interactions.
The Experiment: A Simple Setup, Revolutionary Results
Rutherford's experiment was remarkably simple in its design. A radioactive source emitted alpha particles, which were then collimated into a narrow beam. This beam was directed towards a thin gold foil, only a few atoms thick. A zinc sulfide screen surrounding the foil acted as a detector, producing a scintillation (flash of light) whenever an alpha particle struck it.
The vast majority of alpha particles, as expected based on the plum pudding model, passed straight through the foil, indicating a mostly empty space within the atom. However, a small but significant number of alpha particles were deflected at large angles, and even more surprisingly, a tiny fraction were deflected directly backward.
The Unexpected Backscattering: A Paradigm Shift
The backward scattering of alpha particles was completely inexplicable based on the plum pudding model. The uniform distribution of positive charge predicted only minor deflections. The observed backscattering implied a far more concentrated positive charge within the atom, capable of repelling the positively charged alpha particles with sufficient force to reverse their trajectory.
Analyzing the Data: Uncovering the Atomic Nucleus
Rutherford and his team painstakingly analyzed the scattering data. They observed that:
- Most alpha particles passed straight through: This confirmed the atom's mostly empty nature.
- Some alpha particles were deflected at various angles: This indicated the presence of a concentrated positive charge within the atom.
- A small percentage were deflected back: This strongly suggested a concentrated, massive positive charge capable of exerting a strong repulsive force on the alpha particles.
This led Rutherford to propose a revolutionary new model of the atom: the nuclear model.
The Nuclear Model: A New Atomic Paradigm
Rutherford's nuclear model posited that the atom consists of:
- A tiny, dense, positively charged nucleus: This nucleus contains virtually all the atom's mass and positive charge, concentrated in a region far smaller than the atom's overall size.
- Electrons orbiting the nucleus: The negatively charged electrons orbit the nucleus at a significant distance, accounting for the atom's overall size and its largely empty space.
This model explained the results of the gold foil experiment:
- Straight-through passage: Most alpha particles passed through the atom because the atom is mostly empty space.
- Deflections: Alpha particles passing close to the nucleus experienced a strong repulsive force, resulting in deflections.
- Backscattering: Alpha particles colliding directly with or experiencing a very close encounter with the nucleus experienced a massive repulsive force, leading to backward scattering.
The Force Behind Backscattering: Coulomb's Law
The force responsible for the deflection, and particularly the backscattering, of alpha particles is the electrostatic force described by Coulomb's Law. This law states that the force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
The alpha particle, with its +2e charge, and the nucleus, with its significant positive charge (equal to the atomic number times the elementary charge), exert a strong repulsive force on each other when they are close together. The closer the alpha particle gets to the nucleus, the stronger this repulsive force becomes. For a head-on collision or near miss, this force is sufficient to completely reverse the alpha particle's direction, causing it to scatter backward.
Beyond Gold: Exploring Different Materials
While gold was used in the original experiment due to its malleability and ability to be formed into extremely thin foils, the principles behind alpha particle scattering apply to other materials as well. Experiments using different target materials provided further confirmation of Rutherford's nuclear model and helped to refine our understanding of atomic structure. The scattering patterns observed, analyzed using mathematical models based on Coulomb's Law, provided crucial information about the size and charge of the nucleus of different elements.
Further Refinements and Implications
Rutherford's nuclear model, while revolutionary, wasn't perfect. It didn't explain the stability of the atom (why electrons don't spiral into the nucleus) and didn't account for the then-unknown subatomic particles like neutrons. These limitations were later addressed by advancements in quantum mechanics, leading to the development of more sophisticated models of the atom.
The Enduring Legacy of Rutherford's Experiment
Despite its limitations, Rutherford's gold foil experiment remains a landmark achievement in the history of science. It demonstrated the existence of the atomic nucleus, fundamentally changing our understanding of the structure of matter. The experiment's elegant simplicity and the profound implications of its results highlight the power of scientific inquiry and the importance of questioning established paradigms. The discovery of the backward scattering of alpha particles stands as a testament to the unexpected insights that can be gained from careful observation and rigorous analysis of experimental data. It laid the groundwork for much of our current understanding of nuclear physics and the structure of matter, shaping the future of scientific discovery for generations to come. The seemingly insignificant flash of light on a zinc sulfide screen revealed the universe's deepest secrets – a small but intensely powerful atomic nucleus at the heart of every atom. This groundbreaking experiment remains a cornerstone of modern physics and a reminder of the power of scientific curiosity.
Latest Posts
Latest Posts
-
Whats The Square Root Of 23
Mar 29, 2025
-
What Is The Decimal For 6 10
Mar 29, 2025
-
What Is The Square Root Of 2000
Mar 29, 2025
-
What Are 4 Agents Of Erosion
Mar 29, 2025
-
Molar Mass Of Sr No3 2
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Causes The Alpha Particles To Deflect Backwards . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
