Molar Mass Of Sr No3 2
listenit
Mar 29, 2025 · 5 min read
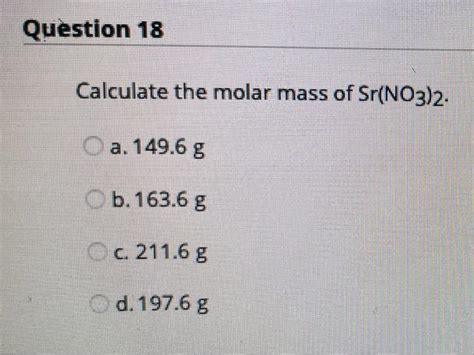
Table of Contents
Determining the Molar Mass of Strontium Nitrate, Sr(NO₃)₂: A Comprehensive Guide
Strontium nitrate, Sr(NO₃)₂, is an inorganic compound with various applications, from pyrotechnics to producing strontium-based materials. Understanding its molar mass is crucial for numerous chemical calculations and experiments. This comprehensive guide will delve into the process of calculating the molar mass of Sr(NO₃)₂, exploring the underlying concepts and providing a step-by-step approach. We'll also discuss the significance of accurate molar mass determination and its implications in various fields.
Understanding Molar Mass
Molar mass, also known as molecular weight, represents the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of elementary entities (atoms, molecules, ions, etc.). The molar mass is expressed in grams per mole (g/mol). Accurate determination of molar mass is vital for stoichiometric calculations, determining the concentration of solutions, and understanding the properties of substances.
Calculating the Molar Mass of Sr(NO₃)₂
Calculating the molar mass of strontium nitrate, Sr(NO₃)₂, involves several steps:
-
Identify the Elements and their Atomic Masses: Sr(NO₃)₂ contains three elements: Strontium (Sr), Nitrogen (N), and Oxygen (O). We need to find their respective atomic masses from the periodic table. The atomic masses are typically given as weighted averages due to the existence of isotopes.
- Strontium (Sr): Approximately 87.62 g/mol
- Nitrogen (N): Approximately 14.01 g/mol
- Oxygen (O): Approximately 16.00 g/mol
-
Determine the Number of Atoms of Each Element: The chemical formula Sr(NO₃)₂ indicates:
- 1 Strontium atom (Sr)
- 2 Nitrogen atoms (2 x N)
- 6 Oxygen atoms (2 x 3 x O)
-
Calculate the Total Mass of Each Element:
- Mass of Sr: 1 atom x 87.62 g/mol = 87.62 g/mol
- Mass of N: 2 atoms x 14.01 g/mol = 28.02 g/mol
- Mass of O: 6 atoms x 16.00 g/mol = 96.00 g/mol
-
Sum the Masses of All Elements: Add the masses of each element to obtain the molar mass of Sr(NO₃)₂:
- Molar Mass of Sr(NO₃)₂ = 87.62 g/mol + 28.02 g/mol + 96.00 g/mol = 211.64 g/mol
Significance of Accurate Molar Mass Determination
The accurate determination of molar mass is crucial in various chemical and related fields:
1. Stoichiometry and Chemical Reactions:
Accurate molar mass values are fundamental to stoichiometric calculations. These calculations help determine the amounts of reactants needed for a specific reaction or the amount of product formed. Inaccurate molar mass values lead to errors in predicting reaction yields and optimizing reaction conditions. For example, in synthesizing other strontium-containing compounds from strontium nitrate, precise molar mass is vital for correct reagent ratios.
2. Solution Chemistry and Concentration Determination:
Molar mass is essential for preparing solutions with specific concentrations. For example, preparing a 1 M (molar) solution of strontium nitrate requires dissolving one mole (211.64 g) of Sr(NO₃)₂ in one liter of solvent. Errors in molar mass will result in incorrect solution concentrations, affecting experimental results.
3. Analytical Chemistry and Quantitative Analysis:
Analytical techniques often rely on molar mass for quantitative analysis. Titrations, gravimetric analysis, and other methods use molar mass to convert measured quantities (like mass or volume) into the amount of substance. This is crucial in determining the purity of compounds, analyzing mixtures, and understanding the composition of materials. In environmental monitoring, for instance, accurate determination of strontium levels in water samples relies on precise molar mass calculations.
4. Physical Chemistry and Material Science:
Molar mass influences several physical properties of materials, including density, melting point, and boiling point. Understanding molar mass is crucial in material science for designing and characterizing new materials with specific properties. For example, in the synthesis of novel strontium-containing ceramics, precise molar mass calculations are vital for achieving the desired material composition.
5. Pharmaceutical and Biomedical Applications:
In pharmaceutical science and related fields, accurate molar mass is critical for dosage calculations, drug formulation, and understanding drug interactions. This is especially true for drugs containing strontium or similar elements, where precise amounts are vital for efficacy and safety.
Potential Sources of Error in Molar Mass Determination
While the calculation of molar mass appears straightforward, potential sources of error exist:
-
Impure Samples: If the strontium nitrate sample used is not pure, the calculated molar mass will be inaccurate. Impurities will add to the overall mass, leading to an overestimation.
-
Inaccurate Atomic Masses: The atomic masses used in the calculation are typically weighted averages and can vary slightly depending on the source. This variability can introduce small errors.
-
Rounding Errors: Rounding off atomic masses during the calculation can accumulate and result in minor discrepancies.
Conclusion
The molar mass of strontium nitrate, Sr(NO₃)₂, is calculated to be 211.64 g/mol. This value is vital for numerous chemical calculations and applications. Accurate determination of molar mass is crucial for stoichiometric calculations, solution preparation, analytical techniques, material science, and various other fields. While the calculation itself is relatively straightforward, it's important to be aware of potential sources of error, such as impure samples or inaccurate atomic masses. Understanding these factors is crucial for ensuring the accuracy and reliability of results in various scientific and technological applications involving strontium nitrate. The precision in molar mass calculation directly impacts the accuracy of numerous experimental results and theoretical predictions. Therefore, meticulous attention to detail in all stages of the calculation process is highly recommended.
Latest Posts
Latest Posts
-
Words Starting With The Same Letter
Mar 31, 2025
-
Does A Rhombus Have 4 Congruent Sides
Mar 31, 2025
-
How To Tell If A Triangle Is Right
Mar 31, 2025
-
What Is The Name Of The Compound Hbr
Mar 31, 2025
-
What Percent Of 36 Is 45
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Molar Mass Of Sr No3 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
