How Many Valence Electrons In P
listenit
Mar 26, 2025 · 5 min read
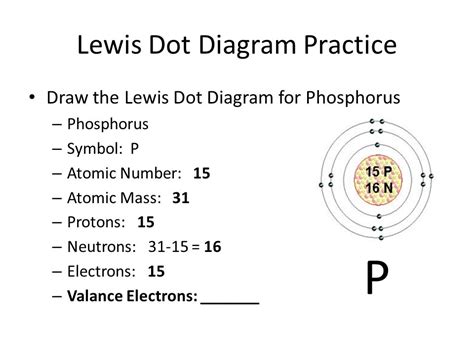
Table of Contents
How Many Valence Electrons in P (Phosphorus)? Understanding Valence Electrons and Their Significance
Phosphorus (P), a crucial element for life, plays a vital role in various biological processes and industrial applications. Understanding its electronic structure, particularly its valence electrons, is key to comprehending its chemical behavior and reactivity. This article delves into the intricacies of valence electrons, specifically focusing on phosphorus and its implications. We'll explore its electron configuration, how to determine the number of valence electrons, and how this impacts its bonding capabilities and chemical properties.
What are Valence Electrons?
Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the furthest from the nucleus and are most loosely bound. They are the primary players in chemical bonding, determining how an atom interacts with other atoms to form molecules and compounds. The number of valence electrons dictates an element's reactivity, its ability to gain, lose, or share electrons to achieve a stable electron configuration, typically resembling a noble gas.
Determining the Number of Valence Electrons
Several methods can be employed to determine the number of valence electrons in an atom. The most common approaches are:
1. Using the Periodic Table:
The periodic table is a powerful tool for quickly determining the number of valence electrons. The group number (vertical column) of an element in the periodic table often directly corresponds to its number of valence electrons.
- Groups 1 and 2 (Alkali and Alkaline Earth Metals): These groups have 1 and 2 valence electrons respectively.
- Groups 13-18 (Boron Group to Noble Gases): The number of valence electrons for these groups can be found by subtracting 10 from the group number. For example, Group 13 elements have 3 valence electrons (13-10 = 3). Group 18 elements, the noble gases, have 8 valence electrons (except helium, which has 2).
- Transition Metals (Groups 3-12): Determining the number of valence electrons for transition metals is more complex, as their electron configuration involves multiple shells and d-orbitals.
2. Using Electron Configuration:
Electron configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. By writing out the electron configuration, we can easily identify the valence electrons.
The electron configuration is built by filling orbitals according to the Aufbau principle (filling lower energy levels first), Hund's rule (maximizing unpaired electrons within a sublevel), and the Pauli exclusion principle (each orbital can hold a maximum of two electrons with opposite spins).
Let's consider the example of phosphorus (P):
Phosphorus has an atomic number of 15, meaning it has 15 protons and 15 electrons in a neutral atom. Its electron configuration is: 1s²2s²2p⁶3s²3p³.
The valence electrons are those in the highest energy level (principal quantum number), which in this case is n=3. This level contains 2 electrons in the 3s subshell and 3 electrons in the 3p subshell. Therefore, phosphorus has a total of 5 valence electrons.
3. Using the Lewis Dot Structure:
Lewis dot structures provide a simplified representation of an atom's valence electrons. The symbol of the element is surrounded by dots, representing each valence electron. For phosphorus (P), the Lewis dot structure would be:
.
. P .
.
The four dots surrounding the "P" symbolize the five valence electrons.
Valence Electrons and the Chemical Behavior of Phosphorus
Phosphorus's five valence electrons significantly impact its chemical behavior and its ability to form various compounds. Because it has five valence electrons and needs eight to achieve a stable octet configuration (like noble gases), phosphorus can:
- Gain three electrons: This would form the phosphide anion (P³⁻), a highly reactive species.
- Share electrons: This is more common and leads to the formation of covalent bonds with other atoms. Phosphorus can form single, double, and even triple bonds depending on the atoms it interacts with. For example, phosphorus forms single bonds with hydrogen in phosphine (PH₃) and multiple bonds in molecules like phosphorus pentachloride (PCl₅).
Examples of Phosphorus Compounds and their Bonding:
- Phosphine (PH₃): Phosphorus forms three single covalent bonds with three hydrogen atoms, utilizing three of its five valence electrons. The remaining two electrons form a lone pair on the phosphorus atom.
- Phosphorus trichloride (PCl₃): Phosphorus forms three single covalent bonds with three chlorine atoms, using three of its valence electrons. The lone pair of electrons remains on the phosphorus atom.
- Phosphorus pentachloride (PCl₅): In this compound, phosphorus forms five covalent bonds with five chlorine atoms. This is an exception to the octet rule because phosphorus can expand its valence shell to accommodate more than eight electrons.
- Phosphoric acid (H₃PO₄): This is a crucial compound in many biological and industrial processes. It features phosphorus with multiple bonds and oxygen atoms.
Importance of Valence Electrons in Various Fields
The understanding of valence electrons is fundamental in numerous fields:
- Chemistry: Predicts chemical reactivity, bond formation, and molecular structures.
- Materials Science: Designing new materials with specific properties, such as semiconductors and catalysts.
- Biology: Understanding the role of phosphorus in biochemical processes, such as DNA and RNA structure and energy transfer (ATP).
- Medicine: Development of new drugs and therapies that target specific molecules based on their electron configurations and bonding properties.
Conclusion:
In summary, phosphorus (P) possesses five valence electrons, as determined through its electron configuration ([Ne]3s²3p³), periodic table group number, and Lewis dot structure. This number significantly dictates its chemical behavior, leading to a wide variety of compounds through the sharing of electrons or the gain of electrons. A firm grasp of valence electrons is essential for comprehending the properties and reactivity of phosphorus, as well as the numerous applications this element holds across diverse scientific and industrial fields. The concept of valence electrons transcends a mere theoretical understanding; it forms the cornerstone of countless chemical reactions and technological advancements. Therefore, mastering this concept is paramount for any aspiring chemist, materials scientist, biologist, or anyone interested in understanding the fundamental building blocks of our world.
Latest Posts
Latest Posts
-
Least Common Multiple 2 And 8
Mar 29, 2025
-
What Is The Greatest Common Factor Of 24 And 4
Mar 29, 2025
-
How Many Light Years Away Is Mars From Earth
Mar 29, 2025
-
Whats The Square Root Of 23
Mar 29, 2025
-
What Is The Decimal For 6 10
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In P . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
