Do Srtrong Bases Completely Dissociate In Water
listenit
Mar 30, 2025 · 5 min read
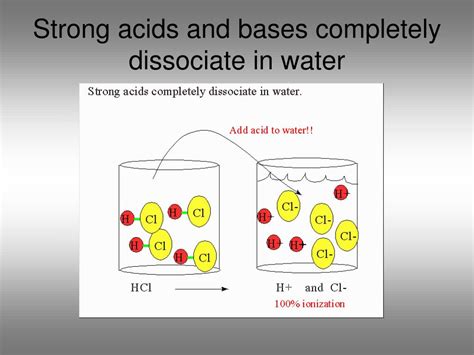
Table of Contents
Do Strong Bases Completely Dissociate in Water? A Deep Dive into Acid-Base Chemistry
The question of whether strong bases completely dissociate in water is a fundamental concept in acid-base chemistry. While the short answer is generally yes, a nuanced understanding requires exploring the complexities of the dissociation process, considering factors like concentration, temperature, and the specific nature of the strong base itself. This article will delve deep into these aspects, providing a comprehensive understanding of strong base dissociation and its implications.
Understanding Strong Bases and Dissociation
Before exploring the intricacies of complete dissociation, let's establish a firm understanding of the key terms involved. A strong base is a substance that readily donates hydroxide ions (OH⁻) when dissolved in water. This donation is essentially a complete transfer of hydroxide ions, leading to a high concentration of OH⁻ ions in the solution. Dissociation, in this context, refers to the separation of a base molecule into its constituent ions in a solvent (water, in this case). Complete dissociation implies that virtually all of the base molecules break apart into their ions.
Examples of Strong Bases
Several common inorganic hydroxides qualify as strong bases. These include:
- Group 1 hydroxides (alkali metal hydroxides): NaOH (sodium hydroxide), KOH (potassium hydroxide), LiOH (lithium hydroxide), etc. These are arguably the most common and readily available strong bases.
- Group 2 hydroxides (alkaline earth metal hydroxides): Ca(OH)₂ (calcium hydroxide), Ba(OH)₂ (barium hydroxide), Sr(OH)₂ (strontium hydroxide). These bases typically exhibit slightly lower solubility in water compared to Group 1 hydroxides, influencing their apparent dissociation.
The Reality of "Complete" Dissociation: It's a Matter of Degree
While the textbook definition often paints a picture of perfect dissociation for strong bases, the reality is slightly more nuanced. The term "complete" is used relatively; it means that the equilibrium of the dissociation reaction lies heavily towards the formation of ions. A tiny fraction of the base might remain undissociated, particularly at higher concentrations.
Consider the dissociation of sodium hydroxide:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
This equation suggests a complete transfer of hydroxide ions. However, at very high concentrations, a minuscule amount of undissociated NaOH molecules might persist. This is because the equilibrium, while strongly favoring dissociation, is not infinitely one-sided.
The Role of Interionic Forces
At higher concentrations, the presence of a significant number of ions in solution leads to stronger interionic forces. These forces can influence the degree of dissociation. The positively charged sodium ions (Na⁺) and negatively charged hydroxide ions (OH⁻) attract each other, creating ion pairs or clusters. While these associations are temporary, they effectively reduce the number of free ions in the solution, slightly diminishing the apparent degree of dissociation.
Temperature's Influence on Dissociation
Temperature also plays a role in the dissociation process. Generally, increasing the temperature will increase the kinetic energy of the molecules, promoting dissociation. Higher kinetic energy helps to overcome the attractive forces between ions, resulting in a more complete dissociation of the strong base. This effect, however, is often minor compared to the influence of concentration.
Practical Implications of (Near) Complete Dissociation
The (near) complete dissociation of strong bases is crucial for several aspects of chemistry and related fields:
-
pH Calculations: The high concentration of hydroxide ions resulting from the dissociation of strong bases allows for straightforward pH calculations. Knowing the concentration of the strong base directly translates to the hydroxide ion concentration, simplifying the calculation of pOH and subsequently pH.
-
Titrations: Strong bases are essential in acid-base titrations, where their complete dissociation ensures accurate determination of the concentration of unknown acids. The sharp endpoint observed in such titrations is a direct consequence of the nearly complete dissociation of the strong base titrant.
-
Industrial Applications: Strong bases find wide applications in various industries. Their complete dissociation allows for their effective use in processes like cleaning, manufacturing, and wastewater treatment. The high concentration of hydroxide ions makes them powerful cleaning agents and aids in various chemical reactions.
-
Solubility of Metal Hydroxides: The extent to which group IIA hydroxides dissolve dictates their usefulness. Even though classified as strong bases, their limited solubility reduces the concentration of free hydroxide ions in solution compared to group IA hydroxides, therefore decreasing the effectiveness.
Comparing Strong Bases with Weak Bases
Understanding the difference between strong and weak bases is critical. Weak bases only partially dissociate in water, meaning a significant portion of the base remains in its molecular form. This results in a much lower concentration of hydroxide ions compared to a strong base of the same concentration. The dissociation of a weak base is described by an equilibrium constant (Kb), which reflects the extent of dissociation. The larger the Kb value, the stronger the weak base. The dissociation of weak bases is significantly impacted by concentration, temperature and the presence of common ions.
Addressing the "Exceptions" – Apparent Incomplete Dissociation
Some situations might appear to contradict the principle of complete dissociation for strong bases. These exceptions are usually related to solubility limitations rather than inherent incomplete dissociation.
For instance, calcium hydroxide, Ca(OH)₂, is considered a strong base because the portion that dissolves dissociates completely. However, its low solubility limits the overall concentration of hydroxide ions in solution. This might lead to a lower than expected pH compared to a more soluble strong base like NaOH at the same nominal concentration. The low solubility is not a characteristic of the base's strength; rather, it’s a factor of its inherent properties.
Similarly, when dealing with highly concentrated solutions of strong bases, the activity of the ions deviates from their concentration due to interionic interactions. These deviations can affect pH measurements and other calculations. Activity coefficients are used to correct for this non-ideality, reflecting the true concentration of active ions.
Conclusion: A Refined Understanding
While the statement that strong bases completely dissociate in water is generally true, it's crucial to acknowledge the nuances. Factors like concentration, temperature, and the specific base's solubility can slightly modify the degree of dissociation. It is more accurate to say that strong bases undergo near-complete dissociation, with minor deviations possible under specific conditions. This understanding is essential for accurate calculations, interpretations, and applications in various fields that utilize strong bases. The concept of complete dissociation, while a powerful simplification, needs to be considered within the context of these factors for a truly comprehensive grasp of acid-base chemistry. This refined understanding allows for more accurate predictions and interpretations in various chemical and practical applications.
Latest Posts
Latest Posts
-
Which Element Is Found In All Organic Compounds
Apr 01, 2025
-
Is Supporting Combustion A Physical Property
Apr 01, 2025
-
100 Cm Equals How Many Meters
Apr 01, 2025
-
Write The Equilibrium Constant Expression For This Reaction
Apr 01, 2025
-
What Is 3 6 In A Fraction
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Do Srtrong Bases Completely Dissociate In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
