Columns On A Periodic Table Are Called
listenit
Mar 24, 2025 · 6 min read
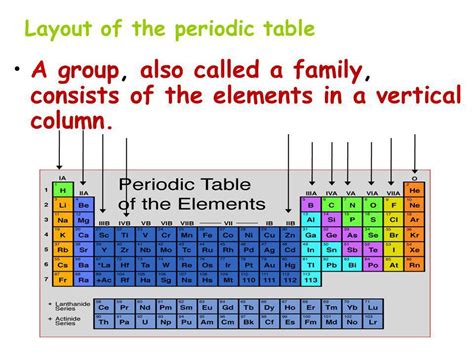
Table of Contents
Columns on a Periodic Table are Called Groups or Families: A Deep Dive
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and resulting properties. Understanding its structure is crucial for comprehending chemical behavior and predicting reactions. One fundamental aspect of this organization is the arrangement of elements into columns, which are formally known as groups or families. This article delves into the significance of these groups, exploring their characteristics, trends, and the underlying reasons for their similarities.
Understanding the Structure of the Periodic Table
Before diving into the specifics of groups, let's briefly review the overall structure of the periodic table. The table arranges elements in a grid, with rows called periods and columns called groups or families. The arrangement is not arbitrary; it reflects the periodic recurrence of similar chemical properties as atomic number increases.
This periodicity is a direct consequence of the arrangement of electrons in electron shells and subshells. Elements within the same group have the same number of valence electrons—the electrons in the outermost shell. These valence electrons are primarily responsible for an element's chemical behavior, determining its reactivity and the types of bonds it can form. This shared number of valence electrons is the key to understanding why elements within the same group exhibit similar properties.
Groups: The Vertical Organization of the Periodic Table
The columns or groups on the periodic table represent families of elements with similar chemical properties. These similarities stem from the fact that elements within the same group have the same number of valence electrons. This shared characteristic leads to predictable patterns in their reactivity, bonding behavior, and physical properties.
There are 18 groups in the standard periodic table, each numbered from 1 to 18. Historically, groups were also designated using Roman numerals and letters (e.g., IA, IIA, VIIA, VIIIA), but the newer numbering system is now more widely adopted.
Key Characteristics of Elements Within a Group
- Similar Chemical Properties: Elements within the same group tend to react similarly with other elements. This is because they have the same number of valence electrons, leading to similar bonding patterns and reactivity.
- Similar Valence Electron Configurations: As mentioned earlier, the most significant similarity is the same number of valence electrons. This governs their tendency to gain, lose, or share electrons during chemical reactions.
- Gradual Trends in Properties: While elements within a group share similarities, there are also gradual changes in their properties as you move down the group. This is due to the increasing number of electron shells and the increasing atomic size. For example, reactivity often increases as you move down a group for alkali metals.
- Predictable Reactivity: The number of valence electrons allows us to predict how an element will react. For instance, elements in Group 1 (alkali metals) readily lose one electron to form +1 ions, while elements in Group 17 (halogens) readily gain one electron to form -1 ions.
Exploring Specific Groups and Their Properties
Let's delve into some of the key groups and their distinctive characteristics:
Group 1: Alkali Metals
This group contains highly reactive metals like lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). They all have one valence electron, making them readily lose this electron to form +1 ions. This explains their high reactivity, especially with water and oxygen. As you move down the group, reactivity increases due to the decreasing ionization energy (the energy required to remove an electron).
Group 2: Alkaline Earth Metals
The alkaline earth metals—beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra)—have two valence electrons. They are also reactive metals, though less so than the alkali metals. They typically form +2 ions by losing their two valence electrons. Similar to alkali metals, their reactivity increases down the group.
Group 17: Halogens
The halogens—fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)—are highly reactive nonmetals. They each have seven valence electrons, meaning they readily gain one electron to achieve a stable octet (eight valence electrons), forming -1 ions. Their reactivity decreases down the group, as the added electron shells shield the nucleus's attraction to the incoming electron.
Group 18: Noble Gases
The noble gases—helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn)—are unique in their extreme unreactivity. They possess a complete valence shell (with the exception of helium, which has a full electron shell), making them exceptionally stable and resistant to forming chemical bonds. This complete valence shell is the reason for their low reactivity and inert nature.
Transition Metals
The transition metals occupy the central block of the periodic table. They are characterized by their variable oxidation states (they can lose different numbers of electrons to form ions with different charges) and the formation of colorful compounds. Their properties are more complex than those of the main group elements due to the involvement of d-electrons in bonding.
Inner Transition Metals (Lanthanides and Actinides)
Located at the bottom of the periodic table, the lanthanides and actinides are also transition metals, but their properties are even more complex due to the involvement of f-electrons in bonding. Many of the actinides are radioactive.
The Importance of Understanding Groups
Understanding the organization of the periodic table into groups is essential for several reasons:
- Predicting Chemical Behavior: Knowing the group of an element allows chemists to predict its reactivity and the types of compounds it will form.
- Designing Chemical Reactions: This knowledge is crucial for designing and controlling chemical reactions, whether in industrial processes, laboratory experiments, or even biological systems.
- Understanding Material Properties: The properties of materials, such as conductivity, strength, and melting point, are directly related to the group an element belongs to.
- Developing New Materials: By understanding the periodic trends within groups, scientists can design new materials with specific properties for various applications.
Conclusion: Groups—A Foundation of Chemical Understanding
The columns on the periodic table, known as groups or families, are not simply a convenient arrangement; they represent a fundamental principle of chemistry. The consistent similarities in properties within each group arise from the shared number of valence electrons, which dictates an element’s chemical behavior. By understanding the characteristics of each group and the trends within them, we can gain invaluable insights into the behavior of elements and the world around us, paving the way for advancements in various fields, from materials science to medicine. The periodic table's organization, especially the understanding of these groups, remains a powerful tool for chemists and scientists alike. The consistent similarities observed within these columns underscore the elegance and predictive power of this fundamental scientific model. Further exploration of individual groups and their unique characteristics will reveal the intricate tapestry of chemical properties and reactivity that the periodic table so elegantly organizes.
Latest Posts
Latest Posts
-
Why Can Ionic Compounds Conduct Electricity
Mar 26, 2025
-
Whats The Diameter Of A Tennis Ball
Mar 26, 2025
-
Why Do Histones Bind Tightly To Dna
Mar 26, 2025
-
Lcm Of 3 5 And 2
Mar 26, 2025
-
What Is The Molar Mass Of Kcl
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Columns On A Periodic Table Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
