Why Can Ionic Compounds Conduct Electricity
listenit
Mar 26, 2025 · 5 min read
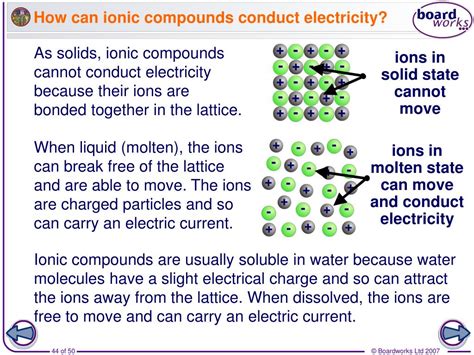
Table of Contents
Why Can Ionic Compounds Conduct Electricity? A Deep Dive into Conductivity
Ionic compounds, with their fascinating interplay of electrostatic forces, exhibit a unique electrical behavior. Unlike many other materials, their ability to conduct electricity isn't straightforward and depends heavily on their physical state. This article delves deep into the microscopic world of ionic compounds to explain why they conduct electricity under specific conditions, exploring the crucial role of ions, charge carriers, and the state of matter.
The Nature of Ionic Compounds: A Sea of Ions
To understand electrical conductivity in ionic compounds, we must first grasp their fundamental structure. Ionic compounds are formed through the electrostatic attraction between oppositely charged ions – cations (positively charged) and anions (negatively charged). This attraction arises from the transfer of electrons from one atom to another, resulting in a stable crystal lattice structure. Think of it as a tightly packed, three-dimensional array of positive and negative ions held together by strong coulombic forces. This structure is the key to understanding their conductivity.
The Role of Ions: The Charge Carriers
The very essence of electrical conductivity lies in the movement of charged particles. In ionic compounds, these charge carriers are the ions themselves. Unlike metals where electrons are delocalized and free to move, ions in a solid ionic compound are locked in their lattice positions. This immobility is the reason why solid ionic compounds are generally poor conductors of electricity.
Crystal Lattice: A Rigid Structure Limiting Mobility
The strong electrostatic forces holding the ions in the crystal lattice prevent them from moving freely. Any attempt to force them to move requires overcoming a significant energy barrier. Consequently, the ions remain essentially immobile in the solid state, preventing the flow of charge and thus limiting electrical conductivity. This is why a solid salt crystal, for example, won't light up a lightbulb.
The Transformative Effect of the Liquid State: Melting Point Magic
The story changes dramatically when we transition from the solid to the liquid state. Melting an ionic compound breaks the rigid crystal lattice, freeing the ions from their fixed positions. In the molten (liquid) state, the ions are no longer constrained and can move relatively freely. This mobility is crucial for electrical conductivity.
Liquid State: Mobility and Conductivity
When an ionic compound melts, the strong electrostatic forces are weakened, allowing the ions to move around. Applying an external electric field then causes these mobile ions to migrate – cations towards the negative electrode (cathode) and anions towards the positive electrode (anode). This directed movement of ions constitutes an electric current, and thus, molten ionic compounds are good conductors of electricity.
Analogy: A Crowd vs. a Flowing River
Imagine a crowd of people (ions) tightly packed in a stadium (solid crystal lattice). Movement is restricted, and the crowd cannot easily flow. Now, imagine the same crowd at a festival (molten state). Individuals can move freely, and if directed, they can flow in a specific direction. The directed flow represents the electric current in a molten ionic compound.
Aqueous Solutions: Dissolution and Conductivity
Another way to achieve conductivity in ionic compounds is by dissolving them in water. When an ionic compound dissolves, the water molecules effectively break apart the crystal lattice, separating the cations and anions. These ions become surrounded by water molecules, a process called hydration, which stabilizes them in solution.
Hydration: Stabilizing Ions in Solution
The hydration of ions helps to overcome the strong electrostatic attraction between them, allowing them to move independently in the aqueous solution. This again provides the mobility necessary for electrical conductivity.
Electrolytes: The Conductivity Powerhouses
Aqueous solutions of ionic compounds are called electrolytes. They conduct electricity because of the presence of mobile ions that can carry charge when an electric field is applied. The conductivity of an electrolyte solution depends on factors like the concentration of the dissolved ions and the mobility of these ions in the solution. Higher concentrations and higher ion mobility lead to higher conductivity.
Examples: Everyday Electrolytes
Numerous everyday substances demonstrate this principle. Table salt (sodium chloride, NaCl) dissolved in water forms an electrolyte, conducting electricity. Similarly, battery acid (sulfuric acid, H₂SO₄), although a covalent compound, ionizes in water to produce mobile ions, making it a strong electrolyte and a key component of batteries.
Factors Affecting Conductivity
Several factors influence the conductivity of ionic compounds, both in their molten and dissolved states:
1. Temperature:
Higher temperatures generally increase conductivity in both molten ionic compounds and aqueous solutions. This is because higher temperatures provide the ions with greater kinetic energy, allowing them to move more rapidly and respond more effectively to an applied electric field.
2. Concentration:
In aqueous solutions, higher concentrations of dissolved ions lead to greater conductivity. More charge carriers (ions) mean more current can flow.
3. Ion Size and Charge:
Smaller ions with higher charges tend to exhibit greater conductivity because they move more easily through the solution or molten state. Larger ions experience greater frictional resistance.
4. Solvent Properties:
In aqueous solutions, the properties of the solvent (water, in this case) play a crucial role. The dielectric constant of the solvent, its ability to reduce the electrostatic attraction between ions, influences the degree of dissociation and hence the conductivity.
Ionic Compounds vs. Metallic Conductors: A Comparison
While both ionic compounds (in molten or dissolved states) and metals conduct electricity, the mechanisms are fundamentally different. In metals, the conductivity is due to the movement of delocalized electrons, which are not bound to specific atoms and are free to move throughout the metallic lattice. In ionic compounds, conductivity is due to the movement of ions, which are charged atoms or molecules.
This difference leads to key distinctions:
- Charge Carriers: Metals: electrons; Ionic Compounds: ions
- Mechanism: Metals: electron flow; Ionic Compounds: ion migration
- Solid State: Metals: good conductors; Ionic Compounds: poor conductors
- Molten/Dissolved State: Metals: remain good conductors; Ionic Compounds: become good conductors
Conclusion: A Versatile Conductivity
The electrical conductivity of ionic compounds is a fascinating aspect of their chemistry. Their unique behavior, contingent on their physical state, highlights the importance of ionic mobility. While solid ionic compounds are poor conductors, melting or dissolving them unleashes the conductive potential of their constituent ions. Understanding this interplay of structure, state, and charge carriers is essential to appreciating the versatile nature of ionic compounds and their diverse applications in various fields, including batteries, electroplating, and many more. The seemingly simple question of "why can ionic compounds conduct electricity?" opens up a world of intricate chemical and physical processes.
Latest Posts
Latest Posts
-
How Many Electron Shells Does Carbon Have
Mar 29, 2025
-
Inverse Function Of X 3 X 2
Mar 29, 2025
-
Why Are Most Fossils Found In Sedimentary Rocks
Mar 29, 2025
-
What Is The Gcf Of 45 And 36
Mar 29, 2025
-
What Number Is 45 Of 90
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Why Can Ionic Compounds Conduct Electricity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
