Columns In The Periodic Table Are Called
listenit
Mar 20, 2025 · 6 min read
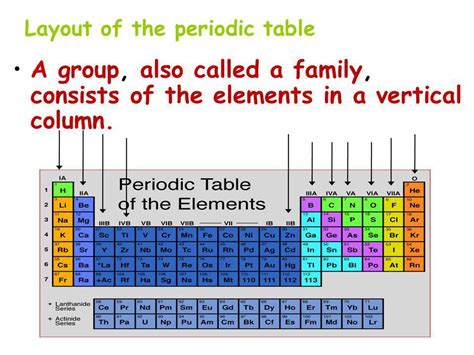
Table of Contents
Columns in the Periodic Table are Called Groups: A Deep Dive into the Organization of Elements
The periodic table, a cornerstone of chemistry, organizes the chemical elements in a structured manner that reveals patterns and relationships in their properties. While the rows are called periods, the columns are called groups or families. Understanding the organization of elements into groups is crucial for predicting their chemical behavior and understanding their properties. This article provides a comprehensive exploration of groups in the periodic table, delving into their characteristics, trends, and significance in chemistry.
What are Groups in the Periodic Table?
Groups in the periodic table represent vertical columns of elements. Elements within the same group share similar chemical properties due to the same number of valence electrons. Valence electrons are the electrons in the outermost shell of an atom, responsible for the atom's ability to form chemical bonds with other atoms. The similarity in valence electron configurations leads to predictable similarities in how these elements react and behave.
Think of it like this: elements in the same group are like members of the same family, sharing similar traits, even though they might have individual differences. Just as members of a family might have different personalities, elements within a group might have slightly different properties, but their core characteristics remain consistent.
The Significance of Group Numbering
The group numbers offer important clues about the electronic configuration and chemical behavior of elements. While different numbering systems exist (e.g., the older IUPAC system and the newer CAS system), the most common system uses numbers 1 through 18. Each group number indicates the number of valence electrons, which helps to determine the element's reactivity and bonding behavior.
For instance:
- Group 1 (Alkali Metals): These elements possess one valence electron, making them highly reactive and prone to losing that electron to form +1 ions.
- Group 18 (Noble Gases): These elements have a full valence shell (eight electrons, except for Helium with two), rendering them exceptionally stable and unreactive.
The group number provides a quick and easy way to categorize elements based on their fundamental chemical characteristics.
Exploring Key Groups and Their Properties
Let's delve deeper into some of the most important groups in the periodic table and examine their characteristic properties:
Group 1: Alkali Metals
- Highly reactive: Their single valence electron is easily lost, forming +1 ions and readily reacting with water and oxygen.
- Soft metals: They are relatively soft and can be easily cut with a knife.
- Low melting points: Compared to other metals, they have relatively low melting points.
- Examples: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), Francium (Fr).
Group 2: Alkaline Earth Metals
- Reactive, but less than alkali metals: They have two valence electrons, making them less reactive than alkali metals.
- Harder and denser: Compared to alkali metals, they are harder and denser.
- Higher melting points: They possess higher melting points than alkali metals.
- Examples: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra).
Group 17: Halogens
- Highly reactive nonmetals: They readily gain one electron to form -1 ions, creating stable compounds.
- Form diatomic molecules: They exist as diatomic molecules (e.g., Cl₂, Br₂) in their elemental form.
- Varied states at room temperature: Fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid.
- Examples: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At).
Group 18: Noble Gases
- Inert: Their full valence electron shells make them extremely unreactive, rarely forming compounds.
- Colorless, odorless gases: They exist as monatomic gases under normal conditions.
- Used in various applications: Helium is used in balloons, neon in lighting, argon in welding, etc.
- Examples: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn).
Transition Metals (Groups 3-12)
- Variable oxidation states: They can exhibit multiple oxidation states, leading to diverse compounds.
- Form colored compounds: Many of their compounds are brightly colored.
- Good conductors of electricity: They are excellent conductors of heat and electricity.
- Examples: Iron (Fe), Copper (Cu), Gold (Au), Silver (Ag), Platinum (Pt).
Trends within Groups
As you move down a group in the periodic table, several trends are observed:
- Atomic radius increases: The size of atoms generally increases as you add electron shells.
- Electronegativity decreases: The tendency of an atom to attract electrons in a chemical bond decreases.
- Ionization energy decreases: The energy required to remove an electron from an atom decreases.
- Metallic character increases: Elements become more metallic in their properties as you go down a group.
These trends are directly related to the increasing number of electron shells and the increasing distance between the valence electrons and the nucleus.
The Importance of Groups in Chemistry
The grouping of elements in the periodic table is fundamental to chemistry for several reasons:
- Predicting properties: Knowing an element's group allows chemists to predict its likely properties and reactivity.
- Understanding chemical reactions: The group membership clarifies how elements will react with each other.
- Designing new materials: The understanding of group properties guides the creation of new materials with specific characteristics.
- Developing chemical technologies: Knowledge of group trends is essential in developing new chemical technologies and processes.
Beyond the Main Groups: Transition Metals and Inner Transition Metals
While the main group elements (Groups 1, 2, and 13-18) are characterized by their consistent valence electron configurations, the transition metals (Groups 3-12) and inner transition metals (lanthanides and actinides) exhibit more complex behaviors.
Transition metals possess partially filled d orbitals, leading to variable oxidation states and complex coordination chemistry. This complexity contributes to their use in a wide range of applications, from catalysis to pigments.
Inner transition metals have partially filled f orbitals, which contribute to their unique magnetic and spectroscopic properties. These elements are crucial in certain technologies, such as nuclear power and specialized lighting.
Conclusion: Groups as the Key to Understanding the Periodic Table
The columns of the periodic table, known as groups or families, are not merely a convenient organizational scheme; they represent a profound reflection of the underlying principles governing the behavior of elements. Understanding the relationships between elements within a group—their shared valence electron configurations, recurring properties, and predictable trends—provides a crucial framework for comprehending the richness and diversity of chemical phenomena. The organization of elements into groups is a testament to the power of periodic law and remains a cornerstone of modern chemistry, essential for researchers, educators, and anyone seeking a deeper appreciation of the fundamental building blocks of matter. The insights gained from studying the groups in the periodic table are indispensable in various scientific and technological advancements. Further exploration into the intricacies of each group reveals the elegance and predictive power embedded within the periodic table's structure.
Latest Posts
Latest Posts
-
How To Convert Rev To Rad
Mar 20, 2025
-
What Is The Most Reactive Metal On The Periodic Table
Mar 20, 2025
-
What Is The Name Of The Compound N2o5
Mar 20, 2025
-
What Is The Sum Of 9260 And 3240
Mar 20, 2025
-
What Is The First Step In Dna Replication
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Columns In The Periodic Table Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
