What Is The Most Reactive Metal On The Periodic Table
listenit
Mar 20, 2025 · 5 min read
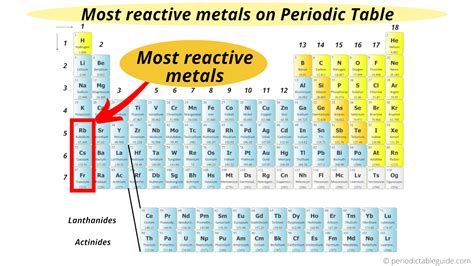
Table of Contents
What is the Most Reactive Metal on the Periodic Table?
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. One key property is reactivity, which describes how readily an element undergoes chemical reactions. While many elements exhibit reactivity, the alkali metals, located in Group 1, are renowned for their exceptionally high reactivity. But which among these metallic titans claims the title of "most reactive"? The answer, while seemingly straightforward, requires a nuanced understanding of reactivity and its measurement. This article delves into the fascinating world of reactive metals, focusing on the alkali metals and the factors contributing to their extreme reactivity. We'll also explore some of the challenges in definitively declaring a single "most reactive" metal.
Understanding Reactivity: More Than Just a Single Reaction
Reactivity isn't a single, easily quantifiable property. It's a complex interplay of several factors, including:
-
Ionization Energy: This refers to the energy required to remove an electron from a neutral atom. Metals, by their nature, readily lose electrons to form positive ions (cations). Lower ionization energies indicate a greater tendency to lose electrons and therefore higher reactivity.
-
Electronegativity: This measures an atom's ability to attract electrons towards itself in a chemical bond. Highly reactive metals have low electronegativity, meaning they're less likely to attract electrons and more likely to donate them.
-
Electropositivity: This is the opposite of electronegativity; it describes the tendency of an atom to lose electrons and form positive ions. Highly reactive metals possess high electropositivity.
-
Standard Reduction Potential: This measures the tendency of a substance to gain electrons (reduction) in a redox reaction. Highly reactive metals have highly negative standard reduction potentials, showing a strong tendency to lose electrons rather than gain them.
-
Atomic Radius: Larger atomic radii generally lead to lower ionization energies and higher reactivity. The outermost electrons are further from the nucleus and experience weaker attraction, making them easier to lose.
The Alkali Metals: The Reactivity Champions
The alkali metals (lithium, sodium, potassium, rubidium, cesium, and francium) occupy Group 1 of the periodic table. Their electronic configuration (ns<sup>1</sup>) means they possess only one electron in their outermost shell. This single valence electron is easily lost, making them incredibly reactive. They readily form +1 ions, reacting violently with water, air, and many other substances.
Comparing the Alkali Metals: A Reactivity Gradient
While all alkali metals are highly reactive, their reactivity increases as you move down the group. This is primarily due to the increasing atomic radius. As you go down the group:
- Atomic radius increases: The outermost electron is further from the nucleus, experiencing weaker electrostatic attraction.
- Ionization energy decreases: Less energy is required to remove the valence electron.
- Electronegativity decreases: The tendency to attract electrons diminishes.
- Electropositivity increases: The tendency to lose electrons increases.
Therefore, cesium generally holds the title of the most reactive alkali metal under standard conditions.
Cesium: The King of Reactivity (Under Standard Conditions)
Cesium's extremely low ionization energy and high electropositivity contribute to its exceptional reactivity. It reacts explosively with water, even more violently than other alkali metals. Exposure to air causes it to ignite spontaneously, forming cesium oxide and superoxide. Its reactions with halogens (fluorine, chlorine, bromine, iodine) are also extremely vigorous and exothermic.
The Case of Francium: A Theoretical Contender
Francium, positioned below cesium in Group 1, theoretically possesses even lower ionization energy and higher reactivity. However, francium is an extremely rare and radioactive element. Its short half-life (approximately 22 minutes for the longest-lived isotope) makes extensive experimentation extremely challenging. While its predicted reactivity surpasses cesium, its scarcity and instability prevent definitive experimental verification.
Factors Affecting Observed Reactivity: Beyond the Periodic Trend
While the general trend of increasing reactivity down Group 1 holds true, several factors can influence observed reactivity in specific reactions:
- Surface area: A larger surface area exposes more atoms to reactants, increasing the reaction rate. A finely divided alkali metal will react more vigorously than a large chunk.
- Temperature: Higher temperatures generally accelerate reaction rates, enhancing the observed reactivity.
- Presence of catalysts: Certain substances can catalyze reactions, altering the reaction pathway and influencing the overall reactivity.
- Specific reactants: The nature of the reacting substance plays a crucial role. The reactivity observed with water will differ from that observed with, for example, a halogen.
Experimental Challenges and the "Most Reactive" Debate
Precisely quantifying and comparing the reactivity of highly reactive metals poses significant experimental challenges:
- Safety concerns: Handling these highly reactive metals requires specialized equipment and meticulous safety precautions to prevent explosions and fires.
- Rapid reaction kinetics: Reactions often occur so quickly that precise measurements are difficult to obtain.
- Unpredictable side reactions: The extreme reactivity can lead to unexpected side reactions, complicating the analysis.
Therefore, declaring a single "most reactive" metal solely based on experimental data is often challenging. The title often depends on the specific reaction being considered and the experimental conditions.
Conclusion: A Complex Question with a Qualified Answer
While cesium generally holds the title of the most reactive metal under standard conditions due to its readily available isotopes and higher abundance compared to Francium, the question of the "most reactive" metal lacks a definitive, universally accepted answer. The inherent complexities of reactivity, experimental limitations, and the influence of various factors contribute to the ongoing discussion. However, understanding the fundamental properties governing reactivity, such as ionization energy and atomic radius, allows for a deeper appreciation of the impressive reactivity of the alkali metals and their unique position within the periodic table's landscape. Further research, including theoretical calculations and refined experimental techniques, may eventually provide a more precise and nuanced understanding of the reactivity scale for these fascinating elements.
Latest Posts
Latest Posts
-
Is Sr Oh 2 A Strong Base
Mar 21, 2025
-
What Is The Absolute Value Of 6
Mar 21, 2025
-
Consider The Trinomial 9x2 21x 10
Mar 21, 2025
-
How Many 1 3 Equal A Cup
Mar 21, 2025
-
How Much Is 1 8 In Grams
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is The Most Reactive Metal On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
