What Is The Name Of The Compound N2o5
listenit
Mar 20, 2025 · 6 min read
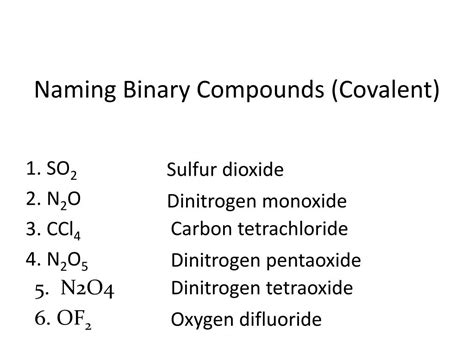
Table of Contents
What is the Name of the Compound N₂O₅?
Dinitrogen pentoxide. That's the name of the compound N₂O₅. But understanding the why behind this name is crucial, not just for memorization, but for a deeper grasp of chemical nomenclature and the properties of this fascinating compound. This article will delve deep into the chemical identity of N₂O₅, exploring its naming conventions, structure, properties, and applications, while also touching on related compounds and its role in various scientific fields.
Understanding Chemical Nomenclature: The System Behind the Name
Before diving into the specifics of N₂O₅, it's essential to understand the basic principles of chemical nomenclature, the system used to name chemical compounds. This system allows chemists worldwide to communicate unambiguously about specific substances. For binary covalent compounds—compounds composed of two non-metal elements—like N₂O₅, the naming convention follows a specific pattern:
-
The first element is named normally. In our case, this is nitrogen (N).
-
The second element is named using its root and the suffix "-ide." Oxygen becomes "oxide."
-
Greek prefixes indicate the number of atoms of each element. The prefixes are: mono- (1), di- (2), tri- (3), tetra- (4), penta- (5), hexa- (6), hepta- (7), octa- (8), nona- (9), and deca- (10). In N₂O₅, we have two nitrogen atoms ("di-") and five oxygen atoms ("penta-").
Therefore, combining these rules, we arrive at the name dinitrogen pentoxide. The systematic naming prevents confusion and ensures clarity in scientific communication.
The Structure and Bonding of Dinitrogen Pentoxide
Dinitrogen pentoxide exists in two forms: a colorless crystalline solid (the most common form) and a gaseous form. Understanding its structure is vital to comprehending its properties and reactivity.
The Solid Form: A Nitrate-Nitronium Salt
The crystalline solid form of dinitrogen pentoxide has a unique structure. It exists as an ionic compound, specifically a salt consisting of nitronium cations (NO₂⁺) and nitrate anions (NO₃⁻). This structure can be represented as [NO₂⁺][NO₃⁻]. The nitronium ion is a linear molecule with a positive charge, while the nitrate ion is a planar triangular molecule with a negative charge. The electrostatic attraction between these oppositely charged ions forms the crystalline lattice.
The Gaseous Form: A Covalent Compound
In the gaseous phase, dinitrogen pentoxide adopts a covalent structure. While its overall empirical formula remains N₂O₅, the bonding arrangement differs significantly from the solid form. The gaseous form exists in equilibrium with its dimer, N₂O₅, and the monomer, NO₂ and NO₃. This dynamic equilibrium makes its gaseous behavior more complex than its solid-state counterpart.
Properties of Dinitrogen Pentoxide
Dinitrogen pentoxide, in its various forms, exhibits a range of distinct properties:
-
Appearance: The solid form is a colorless, crystalline solid. The gaseous form is also colorless.
-
Melting Point: Relatively low melting point for a solid oxide.
-
Solubility: It is soluble in nonpolar solvents, indicating a certain degree of covalent character, especially in its gaseous form.
-
Reactivity: It is a strong oxidizing agent and a powerful nitrating agent. This high reactivity stems from its ability to readily release nitrogen dioxide (NO₂) and other reactive species. This property makes it useful in several applications, as we'll see later.
-
Instability: Dinitrogen pentoxide is relatively unstable, particularly at higher temperatures. It tends to decompose into nitrogen dioxide (NO₂) and oxygen (O₂). This decomposition can be accelerated by exposure to light or moisture.
Synthesis of Dinitrogen Pentoxide
The synthesis of N₂O₅ is typically achieved through various chemical reactions. One common method involves the dehydration of nitric acid (HNO₃) using a dehydrating agent like phosphorus pentoxide (P₄O₁₀). Other methods may involve the reaction of nitrogen dioxide with ozone or other oxidizing agents. The specific process chosen depends on the desired purity and scale of production.
Applications of Dinitrogen Pentoxide
Despite its instability, dinitrogen pentoxide finds applications in several fields:
-
Nitration Reactions: Its strong nitrating ability makes it a key reagent in the production of nitro compounds, which are important intermediates in the synthesis of various chemicals, including explosives, dyes, and pharmaceuticals.
-
Laboratory Reagent: It serves as a useful reagent in various laboratory experiments requiring a strong oxidizing or nitrating agent.
-
Rocket Propellant Research: Although not widely used, its oxidizing capabilities have prompted research into its potential application as a component in rocket propellants.
-
Atmospheric Chemistry: While present in trace amounts in the atmosphere, it plays a role in atmospheric chemistry processes. Its contribution to the formation of other nitrogen oxides and its involvement in ozone depletion reactions are areas of ongoing research.
Safety Precautions when Handling Dinitrogen Pentoxide
Given its reactivity and potential for decomposition, handling dinitrogen pentoxide requires stringent safety precautions:
-
Protective Equipment: Appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat, is essential.
-
Ventilation: Work should be conducted under a well-ventilated fume hood to prevent exposure to toxic gases produced during decomposition.
-
Storage: Store it in a cool, dry, and dark place, away from incompatible materials. Properly sealed containers are crucial to minimize exposure to moisture and prevent decomposition.
-
Waste Disposal: Follow proper waste disposal procedures.
Related Compounds and Comparisons
Understanding dinitrogen pentoxide also involves considering related nitrogen oxides. These include:
-
Nitrogen Dioxide (NO₂): A reddish-brown gas, it is a product of dinitrogen pentoxide decomposition. It is a significant air pollutant.
-
Nitrogen Monoxide (NO): A colorless gas, it is often a precursor to NO₂. It plays a role in various biological processes.
-
Nitrous Oxide (N₂O): Also known as "laughing gas," it is a potent greenhouse gas and anesthetic.
Comparing these compounds highlights the range of properties and reactivity within the nitrogen oxides family. The oxidation state of nitrogen and the number of oxygen atoms greatly influence these properties.
Ongoing Research and Future Applications
Research on dinitrogen pentoxide continues, particularly in areas such as:
-
Improved synthesis methods: Developing more efficient and sustainable methods for producing high-purity dinitrogen pentoxide.
-
Catalysis: Investigating its role as a catalyst or co-catalyst in various chemical reactions.
-
Atmospheric chemistry modeling: Improving the accuracy of atmospheric models incorporating its reactions and contribution to environmental processes.
These research efforts may lead to new applications and a better understanding of this fascinating compound's role in both industrial and environmental settings.
Conclusion
Dinitrogen pentoxide (N₂O₅), while not a household name, plays a significant role in chemistry and related fields. Its systematic name, derived from established nomenclature rules, clearly identifies its composition. Its unique structure, properties, reactivity, and potential applications make it a compound of considerable scientific interest. Understanding its synthesis, handling, and safety precautions is crucial for researchers and those working with it. Ongoing research will undoubtedly continue to uncover new insights and potential uses for this remarkable chemical.
Latest Posts
Latest Posts
-
X 3 3x 2 4x 12
Mar 21, 2025
-
What Is The Lowest Common Multiple Of 15 And 20
Mar 21, 2025
-
Can Crawfish Live Out Of Water
Mar 21, 2025
-
Whats The Difference Between Static And Current Electricity
Mar 21, 2025
-
Least Common Multiple Of 3 8
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is The Name Of The Compound N2o5 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
