Can Mixtures Be Separated By Physical Means
listenit
Mar 25, 2025 · 6 min read
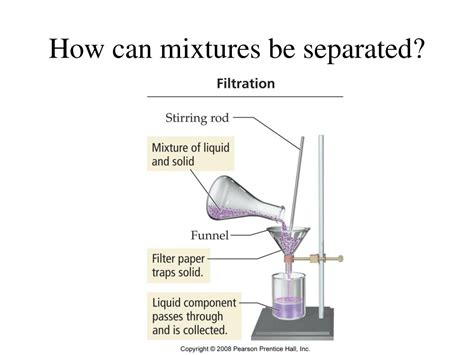
Table of Contents
Can Mixtures Be Separated by Physical Means? A Comprehensive Guide
Many substances in the world around us exist as mixtures—combinations of two or more components that are not chemically bonded. Understanding how mixtures are formed and, crucially, how they can be separated is fundamental to various scientific disciplines and everyday life. This comprehensive guide delves into the fascinating world of mixture separation using solely physical methods, exploring different techniques and their applications.
What is a Mixture?
Before diving into separation techniques, it's crucial to define what constitutes a mixture. A mixture is a physical combination of two or more substances whose identities are retained when mixed in solutions, suspensions, and colloids. Unlike compounds, where substances are chemically bonded, mixtures can be separated by physical means without altering the chemical nature of their components. This is because the components retain their individual properties within the mixture.
There are three main types of mixtures:
-
Homogeneous Mixtures: These mixtures have a uniform composition throughout. Examples include saltwater, air (a mixture of gases), and sugar dissolved in water. The individual components are indistinguishable to the naked eye.
-
Heterogeneous Mixtures: These mixtures have a non-uniform composition, with visibly distinct components. Examples include sand and water, oil and water, and a salad. The different components are easily identifiable.
-
Colloids: These represent an intermediate state between homogeneous and heterogeneous mixtures. They appear homogeneous to the naked eye but are microscopically heterogeneous. The dispersed particles are larger than those in a solution but small enough to remain suspended. Examples include milk, fog, and blood.
Physical Methods of Separating Mixtures
The beauty of separating mixtures using physical methods lies in the simplicity and preservation of the components' chemical properties. Several techniques exist, each tailored to the specific characteristics of the mixture:
1. Filtration
Filtration is a widely used method for separating heterogeneous mixtures consisting of a solid and a liquid. This technique involves passing the mixture through a porous material, such as filter paper or a sieve. The solid particles are trapped by the filter, while the liquid passes through, forming the filtrate. This method is effective for separating sand from water, separating precipitates from a solution after a chemical reaction, and many other applications in chemistry and everyday life, such as using a coffee filter.
Applications:
- Water purification: Removing sediments and impurities from water.
- Laboratory experiments: Isolating solid products from reactions.
- Industrial processes: Separating solids from liquids in various manufacturing processes.
2. Decantation
Decantation is a simple technique used to separate a mixture of liquid and solid or two immiscible liquids (liquids that don't mix). It involves carefully pouring off the liquid, leaving the solid or denser liquid behind. This method works best when the solid has settled to the bottom or the liquids form distinct layers. It's a less precise method than filtration but requires minimal equipment.
Applications:
- Separating sand and water: Letting the sand settle and pouring off the water.
- Separating oil and water: Allowing the less dense oil to float to the top and carefully removing it.
- Winemaking: Removing sediment from wine.
3. Evaporation
Evaporation is used to separate a soluble solid from a liquid solution. The solution is heated, causing the liquid solvent to evaporate, leaving behind the solid solute. This method is commonly used to obtain salt from saltwater. The rate of evaporation can be increased by increasing the surface area of the liquid or by using a fan.
Applications:
- Salt production: Obtaining salt from seawater.
- Crystallization: Producing pure crystals of a solid solute.
- Preparing extracts: Removing solvents from plant extracts.
4. Distillation
Distillation is a more sophisticated technique used to separate mixtures of liquids with different boiling points. The mixture is heated, and the component with the lower boiling point vaporizes first. The vapor is then condensed back into a liquid and collected separately. This process is repeated to achieve higher levels of purification. Fractional distillation is used for separating liquids with boiling points that are closer together.
Applications:
- Crude oil refining: Separating different components of crude oil based on their boiling points.
- Water purification: Producing distilled water by removing impurities.
- Alcohol production: Separating ethanol from fermented solutions.
5. Chromatography
Chromatography is a powerful separation technique based on the differential distribution of components between a stationary phase and a mobile phase. Different components travel at different rates through the stationary phase, allowing for their separation. Paper chromatography, thin-layer chromatography (TLC), and column chromatography are common variations. This is widely used in analytical chemistry and biochemistry for identifying and purifying substances.
Applications:
- Forensic science: Analyzing inks and dyes.
- Biochemistry: Separating and identifying proteins and other biomolecules.
- Environmental science: Analyzing pollutants in water or air.
6. Magnetism
Magnetism is a simple but effective method for separating mixtures containing magnetic materials. A magnet is used to attract and remove the magnetic component from the mixture. This is commonly used for separating iron filings from sand or other non-magnetic materials.
Applications:
- Recycling: Separating iron and steel from other scrap materials.
- Industrial processes: Removing magnetic impurities from materials.
- Laboratory experiments: Separating magnetic substances from mixtures.
7. Centrifugation
Centrifugation utilizes centrifugal force to separate mixtures containing solid particles suspended in a liquid or two immiscible liquids with different densities. The mixture is spun at high speed in a centrifuge, causing the denser components to sediment at the bottom, while the lighter components remain at the top.
Applications:
- Blood separation: Separating blood components (red blood cells, white blood cells, plasma).
- Dairy industry: Separating cream from milk.
- Laboratory experiments: Separating cells or subcellular components.
8. Sublimation
Sublimation is a less commonly used method that involves separating mixtures of solids where one component can directly transition from a solid to a gas phase without becoming a liquid. The gas is then cooled and collected as a solid. This is useful for separating substances with different sublimation properties, such as separating iodine from sand.
Applications:
- Purification of iodine and other sublimatory substances.
- Separating solids with different sublimation points.
Choosing the Appropriate Separation Technique
The selection of the most appropriate separation technique depends largely on the nature of the mixture. Factors such as the physical states of the components (solid, liquid, gas), their solubility, boiling points, and magnetic properties all influence the choice of method. Often, a combination of techniques might be necessary to achieve complete separation. For example, separating a mixture of sand, salt, and iron filings might require the use of a magnet (iron), followed by filtration (sand), and finally, evaporation (salt).
Conclusion
The separation of mixtures using physical methods is a cornerstone of various scientific and industrial processes. Understanding the principles underlying each technique—filtration, decantation, evaporation, distillation, chromatography, magnetism, centrifugation, and sublimation—is essential for anyone working in a scientific or technical field. The choice of method hinges on the specific characteristics of the mixture, and often, a combination of techniques is employed to achieve complete and efficient separation. The beauty of these techniques is their ability to separate components without altering their chemical identities, preserving their properties for further analysis or use. This understanding facilitates advancements in various areas, from material science to environmental remediation.
Latest Posts
Latest Posts
-
What Is The Formula For The Compound Magnesium Oxide
Mar 28, 2025
-
What Is The Correct Formula For Calcium Oxide
Mar 28, 2025
-
What Is The Si Base Unit Of Length
Mar 28, 2025
-
What Is The Oxidation State Of Each Element In Coh2
Mar 28, 2025
-
How Many Valence Electrons Are In Boron
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Can Mixtures Be Separated By Physical Means . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
