Aufbau Principle Hund's Rule Pauli Exclusion
listenit
Mar 25, 2025 · 6 min read
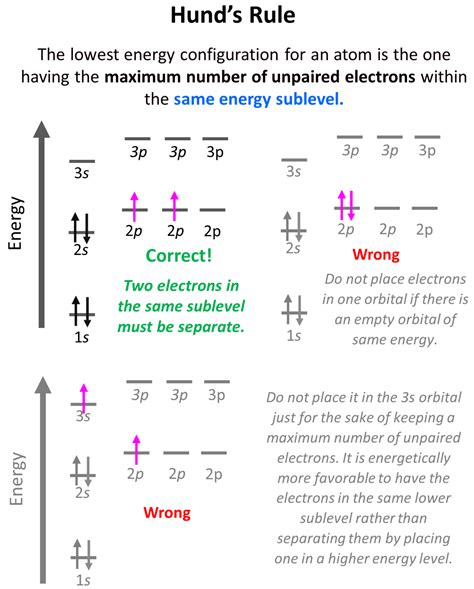
Table of Contents
Understanding Atomic Structure: Aufbau Principle, Hund's Rule, and Pauli Exclusion Principle
The arrangement of electrons within an atom dictates its chemical properties and reactivity. Understanding how electrons populate atomic orbitals is crucial to comprehending the behavior of elements and the formation of molecules. This understanding hinges on three fundamental principles: the Aufbau principle, Hund's rule, and the Pauli exclusion principle. These principles, while seemingly simple, provide the framework for predicting electron configurations and interpreting the periodic table's structure.
The Aufbau Principle: Filling Orbitals in Order of Increasing Energy
The Aufbau principle, derived from the German word "Aufbau" meaning "building up," dictates that electrons fill atomic orbitals in order of increasing energy levels. This sequential filling ensures the atom achieves the lowest possible energy state, also known as the ground state. The order of orbital filling is not simply 1s, 2s, 2p, 3s, 3p, and so on. The energies of orbitals can be influenced by factors like electron-electron repulsion, leading to some exceptions.
Energy Level Diagram and Orbital Filling
A crucial tool for visualizing this process is the energy level diagram. This diagram shows the relative energies of different atomic orbitals. Orbitals with lower energy are filled first before higher energy orbitals. The order generally follows:
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p…
Note: The (n+l) rule can help predict the filling order. 'n' represents the principal quantum number, and 'l' represents the azimuthal quantum number (0 for s, 1 for p, 2 for d, 3 for f). The orbital with the lower (n+l) value fills first; if (n+l) values are equal, the orbital with the lower 'n' value fills first.
Exceptions to the Aufbau Principle
While the Aufbau principle provides a general guideline, exceptions exist, particularly in transition metals and lanthanides/actinides. These exceptions often arise due to the relatively small energy difference between certain orbitals. For instance, chromium (Cr) and copper (Cu) have electron configurations that deviate from the expected pattern due to the enhanced stability of half-filled and fully filled subshells. These deviations result from the balance between minimizing electron-electron repulsion and maximizing orbital stability.
Hund's Rule: Maximizing Spin Multiplicity
Hund's rule, also known as Hund's rule of maximum multiplicity, addresses how electrons populate degenerate orbitals (orbitals with the same energy level within a subshell). It states that electrons will individually occupy each orbital within a subshell before pairing up in any one orbital. Furthermore, these unpaired electrons will have parallel spins (the same spin quantum number, either +1/2 or -1/2).
Importance of Parallel Spins
The parallel spins of unpaired electrons are crucial for minimizing electron-electron repulsion. By occupying separate orbitals with parallel spins, the electrons are spatially further apart, leading to a lower energy state compared to pairing up in a single orbital. This results in greater stability for the atom.
Application of Hund's Rule
Consider the nitrogen atom (N), which has seven electrons. The electron configuration is 1s²2s²2p³. According to Hund's rule, the three electrons in the 2p subshell will each occupy a separate 2p orbital with parallel spins before pairing up. This maximizes the total spin of the atom and lowers the overall energy.
Pauli Exclusion Principle: Distinguishing Electrons within an Atom
The Pauli exclusion principle is a cornerstone of quantum mechanics, stating that no two electrons within an atom can have the same set of four quantum numbers. These four quantum numbers are:
- Principal quantum number (n): Describes the energy level and size of the orbital (n = 1, 2, 3…).
- Azimuthal quantum number (l): Describes the shape of the orbital (l = 0, 1, 2… n-1; s, p, d, f…).
- Magnetic quantum number (ml): Describes the orientation of the orbital in space (ml = -l, …, 0, …, +l).
- Spin quantum number (ms): Describes the intrinsic angular momentum of the electron (ms = +1/2 or -1/2; spin up or spin down).
Implications of the Pauli Exclusion Principle
The Pauli exclusion principle directly limits the number of electrons that can occupy a particular orbital. Since only two possible values exist for the spin quantum number (ms), each orbital can hold a maximum of two electrons, with opposite spins. This principle is fundamental to the structure of the periodic table and the behavior of atoms. It explains why elements exhibit specific chemical properties based on their electron configurations.
Combining the Principles: Predicting Electron Configurations
Applying the Aufbau principle, Hund's rule, and the Pauli exclusion principle together allows for the prediction of electron configurations for various atoms. This involves systematically filling orbitals according to their increasing energy, maximizing the number of unpaired electrons with parallel spins, and ensuring that no two electrons share the same set of four quantum numbers.
Example: Determining the Electron Configuration of Oxygen
Let's determine the electron configuration of oxygen (O), which has eight electrons:
- Aufbau Principle: We fill orbitals in order of increasing energy: 1s, 2s, 2p.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons with opposite spins.
- Hund's Rule: Electrons fill orbitals individually before pairing up, and unpaired electrons have parallel spins.
Therefore, the electron configuration of oxygen is 1s²2s²2p⁴. The four 2p electrons occupy the three 2p orbitals as follows: two electrons pair up in one 2p orbital, and the remaining two electrons each occupy a separate 2p orbital with parallel spins.
Applications and Importance of these Principles
These three principles are not merely abstract concepts; they are fundamental to understanding a vast range of phenomena in chemistry and physics.
Understanding Chemical Bonding
The electron configurations determined using these principles are crucial for understanding chemical bonding. Atoms interact to achieve a more stable electron configuration, often by filling their valence shells. This drive for stability underlies the formation of ionic, covalent, and metallic bonds.
Predicting Reactivity
The reactivity of an element is directly related to its electron configuration, particularly the number of electrons in its outermost shell (valence electrons). Elements with partially filled valence shells tend to be more reactive than those with completely filled or empty valence shells.
Explaining Periodic Trends
The periodic table's organization reflects the electron configurations of elements. Periodic trends, such as electronegativity, ionization energy, and atomic radius, are directly linked to electron arrangements predicted by the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
Spectroscopy and Atomic Structure
Spectroscopic techniques provide experimental evidence supporting these principles. By analyzing the absorption and emission spectra of atoms, scientists can determine electron transitions between different energy levels, which directly relate to the orbital filling predicted by these fundamental principles.
Conclusion: Building Blocks of Atomic Structure
The Aufbau principle, Hund's rule, and the Pauli exclusion principle are essential pillars in understanding atomic structure and electron configurations. These principles provide a framework for predicting how electrons are arranged within atoms, explaining their properties, and driving their interactions. Their applications extend across various fields, impacting our understanding of chemical bonding, reactivity, periodic trends, and spectroscopic analyses. Mastering these principles is crucial for anyone studying chemistry, physics, or related fields. Their seemingly simple rules underpin the complexity and beauty of the atomic world.
Latest Posts
Latest Posts
-
What Is Half A Mile In Feet
Mar 26, 2025
-
What Percent Of 12 5 Is 39
Mar 26, 2025
-
Empirical And Molecular Formula Of Ibuprofen
Mar 26, 2025
-
What Is The Molar Mass Of So2
Mar 26, 2025
-
Electrons In The Outermost Energy Level Are Called
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Aufbau Principle Hund's Rule Pauli Exclusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
