Are Liquids Included In Equilibrium Constant
listenit
Apr 01, 2025 · 5 min read
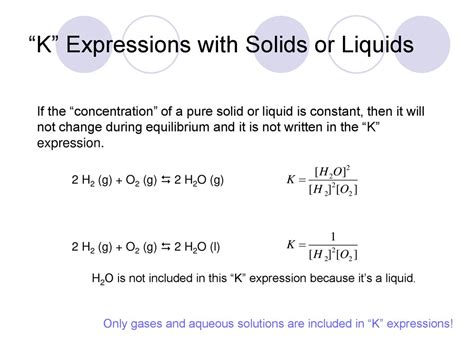
Table of Contents
Are Liquids Included in the Equilibrium Constant? A Deep Dive into Chemical Equilibrium
Understanding chemical equilibrium is crucial in chemistry, and a common point of confusion revolves around the inclusion of liquids (and solids) in the equilibrium constant expression, K. This comprehensive guide will delve into this topic, clarifying the nuances and providing a robust understanding of the principles at play.
Understanding the Equilibrium Constant (K)
Before addressing the specifics of liquids, let's establish a firm foundation in the concept of the equilibrium constant. The equilibrium constant, K, is a numerical value that describes the ratio of products to reactants at equilibrium for a reversible reaction at a given temperature. It's a powerful tool that allows us to predict the direction a reaction will proceed under specific conditions and the relative amounts of reactants and products present at equilibrium.
For a generic reversible reaction:
aA + bB ⇌ cC + dD
Where a, b, c, and d represent the stoichiometric coefficients, the equilibrium constant expression is:
K = ([C]<sup>c</sup>[D]<sup>d</sup>) / ([A]<sup>a</sup>[B]<sup>b</sup>)
The bracketed terms represent the molar concentrations of each species at equilibrium.
The Role of Activities in Equilibrium
The true, thermodynamically rigorous definition of the equilibrium constant involves activities rather than concentrations. Activity is a measure of the "effective concentration" of a species, accounting for deviations from ideal behavior. For dilute solutions, activity approximates concentration. However, for non-ideal solutions or for pure solids and liquids, the activity takes on a different meaning.
For ideal solutions or dilute solutions: Activity ≈ Concentration
For pure solids and liquids: Activity = 1
This is the key to understanding why pure solids and liquids are not explicitly included in the equilibrium constant expression. Since their activity is always 1, including them in the expression would not change the numerical value of K.
Why Are Pure Liquids and Solids Excluded from K?
The exclusion of pure liquids and solids from the equilibrium constant expression stems from the fact that their concentrations remain essentially constant throughout the reaction. Consider a reaction involving a pure liquid or solid reactant or product: the concentration of the pure liquid or solid is determined by its density, which remains virtually unchanged during the reaction, provided that the quantity of the solid or liquid does not change significantly.
Example: Consider the dissolution of calcium carbonate in water:
CaCO₃(s) ⇌ Ca²⁺(aq) + CO₃²⁻(aq)
The equilibrium constant expression is:
K<sub>sp</sub> = [Ca²⁺][CO₃²⁻]
Notice that CaCO₃(s), being a pure solid, is not included in the expression. Its activity is 1, and including it would not affect the value of K<sub>sp</sub> (the solubility product constant).
Impact of Solvent Concentration
The solvent in a reaction mixture deserves special consideration. For reactions in dilute solutions where the solvent is water, the concentration of water remains essentially constant throughout the reaction and is therefore also not explicitly included in the equilibrium constant expression. The activity of water is approximately 1.
However, if the solvent concentration changes significantly (e.g., in highly concentrated solutions or reactions in non-aqueous solvents where the solvent participates directly in the reaction), the solvent’s concentration might need to be considered in the equilibrium constant expression, modifying the expression accordingly.
Equilibrium Constants and Different Phases
Reactions often involve multiple phases (e.g., solid-liquid, gas-liquid). This further underscores the importance of activity. For pure solids and liquids, activity remains constant (1), while the activities of gaseous and aqueous species are usually approximated by their partial pressures (for gases) and concentrations (for aqueous species).
The equilibrium constant expression, therefore, reflects the activities of only the species whose concentrations can change during the reaction.
Examples Illustrating the Exclusion of Liquids and Solids
Let's illustrate the principle with a few more examples:
1. Esterification Reaction:
The reaction between an acid and an alcohol to form an ester and water:
CH₃COOH(l) + CH₃CH₂OH(l) ⇌ CH₃COOCH₂CH₃(l) + H₂O(l)
The equilibrium constant expression is:
K = [CH₃COOCH₂CH₃][H₂O] / [CH₃COOH][CH₃CH₂OH]
Although all components are liquids, this is a simplified expression that assumes ideal behavior. In reality, the activity of each liquid component should be considered, though in practice this is often approximated by concentration.
2. Dissolution of a Gas in a Liquid:
Consider the dissolution of carbon dioxide in water:
CO₂(g) ⇌ CO₂(aq)
The equilibrium constant expression (Henry's Law) usually focuses on the partial pressure of CO₂ and the concentration of dissolved CO₂:
K<sub>H</sub> = [CO₂(aq)] / P<sub>CO₂</sub>
Water, as the solvent, is not included, assuming dilute aqueous solution.
3. Decomposition of Calcium Carbonate:
The thermal decomposition of calcium carbonate:
CaCO₃(s) ⇌ CaO(s) + CO₂(g)
The equilibrium constant expression only includes the partial pressure of CO₂:
K<sub>p</sub> = P<sub>CO₂</sub>
Both CaCO₃(s) and CaO(s) are pure solids and have an activity of 1.
When to Include Solvent Concentration
We've emphasized the typical exclusion of solvent concentration. But there are exceptions.
- Non-Dilute Solutions: In highly concentrated solutions, the solvent's activity deviates significantly from unity, requiring its inclusion in the equilibrium constant expression. This often involves more complex activity coefficient calculations.
- Solvent Participation: If the solvent directly participates in the reaction mechanism, its concentration must be included in the expression. This is less common but possible in reactions involving specific solvent interactions.
- Reactions in Non-Aqueous Solvents: Reactions in solvents other than water often require more careful consideration of solvent activity, particularly if the solvent concentration changes significantly during the reaction.
Conclusion: A Practical Perspective
While the thermodynamically rigorous definition of K involves activities, in practice, using concentrations (for dilute solutions of gases and aqueous species) and omitting pure solids and liquids often provides accurate predictions of the equilibrium position. The exclusion of pure solids, liquids (except under specific conditions as outlined above) simplifies the equilibrium constant expression without sacrificing accuracy in many common scenarios. However, remember that this simplification relies on the assumption of ideal or near-ideal behavior. Understanding the underlying principles of activity and its relation to concentration provides a crucial foundation for correctly interpreting and using equilibrium constants. For non-ideal conditions, more advanced treatments are required, often using activity coefficients to account for deviations from ideality. Understanding this distinction provides a solid grounding in chemical equilibrium and ensures accurate and meaningful interpretation of experimental data.
Latest Posts
Latest Posts
-
What Is 3 Divided By 11
Apr 02, 2025
-
How To Find The Mass Of The Excess Reactant
Apr 02, 2025
-
Instantaneous Rate Of Change Vs Average Rate Of Change
Apr 02, 2025
-
How Many D Orbitals Can Be In An Energy Level
Apr 02, 2025
-
Log Base 2 X 2 Graph
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Are Liquids Included In Equilibrium Constant . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
