Why Lipids Are Not Soluble In Water
listenit
Mar 20, 2025 · 5 min read
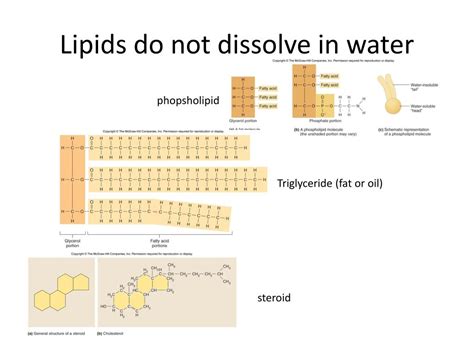
Table of Contents
Why Lipids Are Not Soluble in Water: A Deep Dive into Hydrophobicity
Lipids, a diverse group of naturally occurring molecules, are largely defined by their insolubility in water. This characteristic, known as hydrophobicity, is fundamental to their structure, function, and biological roles. Understanding why lipids are not soluble in water requires a close examination of their chemical structure and the interactions between molecules and water. This article will explore the reasons behind lipid insolubility, examining the different types of lipids and the forces that govern their behavior in aqueous environments.
The Nature of Water and Hydrophilic Interactions
Before delving into the hydrophobic nature of lipids, let's understand the properties of water. Water is a highly polar molecule, meaning it has an uneven distribution of charge. The oxygen atom carries a partial negative charge (δ-), while the hydrogen atoms carry partial positive charges (δ+). This polarity allows water molecules to form strong hydrogen bonds with each other and with other polar molecules. These hydrogen bonds are responsible for water's high surface tension, boiling point, and its ability to act as a universal solvent for many polar substances.
Substances that readily dissolve in water are called hydrophilic. Their solubility stems from their ability to interact favorably with water molecules through hydrogen bonding, dipole-dipole interactions, or ion-dipole interactions. These interactions are energetically favorable, outweighing the energy required to separate water molecules to create space for the solute. Examples of hydrophilic molecules include sugars, amino acids, and many salts.
The Chemical Structure of Lipids and Hydrophobic Interactions
In contrast to hydrophilic molecules, lipids are primarily composed of carbon and hydrogen atoms, with relatively few oxygen atoms. This hydrocarbon backbone is largely nonpolar, meaning it has a uniform distribution of charge. This nonpolarity is the key reason for their insolubility in water.
The interaction between nonpolar molecules and water is unfavorable. Water molecules, in their desire to maximize hydrogen bonding, tend to cluster around nonpolar substances, forming a "cage-like" structure. This highly ordered structure reduces the entropy (disorder) of the system, making the interaction energetically unfavorable. To minimize this unfavorable interaction, nonpolar molecules tend to aggregate together, minimizing their contact with water. This phenomenon is known as the hydrophobic effect.
Types of Lipids and their Hydrophobic Properties
The hydrophobic nature of lipids is consistent across various lipid classes, though the extent of hydrophobicity may vary slightly depending on the specific structure. Let's explore some major lipid classes:
1. Fatty Acids: These are long hydrocarbon chains with a carboxyl group (-COOH) at one end. The long hydrocarbon tail is highly nonpolar, responsible for their insolubility in water. The carboxyl group is polar, but its influence is overshadowed by the long hydrophobic tail.
2. Triglycerides: These are esters formed from glycerol and three fatty acids. The glycerol backbone is relatively small and polar, but the three fatty acid tails dominate, making triglycerides highly hydrophobic and insoluble in water. Their insolubility contributes to their role in energy storage.
3. Phospholipids: These are crucial components of cell membranes. They consist of a glycerol backbone, two fatty acid tails (hydrophobic), and a phosphate group linked to a polar head group (hydrophilic). This amphipathic nature (having both hydrophilic and hydrophobic regions) is critical for the formation of bilayers, with the hydrophobic tails facing inward and the hydrophilic heads interacting with the aqueous environment. While the overall molecule is amphipathic, the significant hydrophobic portion contributes to their limited solubility in water.
4. Steroids: These lipids have a characteristic four-ring structure. Steroids, such as cholesterol, have both polar and nonpolar regions, making them amphipathic. Although they possess some polar groups, their overall structure makes them only slightly soluble in water.
5. Waxes: These are esters formed from a long-chain fatty acid and a long-chain alcohol. Their extensive hydrocarbon chains make them highly hydrophobic and insoluble in water. Their insolubility is crucial for their protective functions in plants and animals.
The Hydrophobic Effect: A Deeper Look
The hydrophobic effect isn't simply the repulsion between nonpolar molecules and water; it's a more complex phenomenon driven by the maximization of entropy in the aqueous environment. When nonpolar molecules are introduced into water, they disrupt the hydrogen bonding network, forcing water molecules to reorganize into a more ordered structure around them. This decrease in entropy is energetically unfavorable.
The aggregation of nonpolar molecules reduces the surface area exposed to water, minimizing the disruption of the hydrogen bonding network and increasing the overall entropy of the system. This increase in entropy is the driving force behind the hydrophobic effect. It's not that nonpolar molecules are actively repelled by water; it's that their presence forces water into a less favorable, more ordered state, which the system tries to avoid.
Implications of Lipid Insolubility in Biological Systems
The insolubility of lipids in water has profound implications for biological systems. Their hydrophobic nature allows them to perform crucial functions:
- Cell Membrane Structure: The amphipathic nature of phospholipids enables the formation of the cell membrane, separating the internal cellular environment from the external surroundings.
- Energy Storage: Triglycerides, due to their high energy density and insolubility, serve as efficient energy storage molecules in adipose tissue.
- Hormone Production: Steroid hormones, despite their limited solubility, are essential signaling molecules regulating various physiological processes.
- Protection and Insulation: Waxes provide a protective waterproof coating on plant leaves and animal fur.
Techniques to Handle Lipid Solubility Challenges
Because of their insolubility, special techniques are required to work with lipids in laboratory settings. These include:
- Use of organic solvents: Lipids are readily soluble in nonpolar organic solvents like chloroform, ether, and hexane.
- Emulsification: Lipids can be dispersed in water by forming emulsions, using emulsifying agents that have both hydrophilic and hydrophobic regions.
- Liposomes: Artificial vesicles can be created from phospholipids, providing a way to encapsulate and deliver hydrophobic drugs or other molecules.
Conclusion
The insolubility of lipids in water, stemming from their largely nonpolar hydrocarbon structures, is not a mere chemical quirk but a fundamental property with profound biological consequences. The hydrophobic effect, a complex interplay of entropy and intermolecular forces, drives the self-assembly of lipids into crucial structures like cell membranes and influences their roles in energy storage, signaling, and protection. Understanding lipid hydrophobicity is essential for comprehending cellular processes, developing new technologies, and advancing various fields of biological research. Further investigation into the complexities of lipid-water interactions promises to reveal even more about the intricacies of life itself.
Latest Posts
Latest Posts
-
How Much Feet Is 168 Cm
Mar 21, 2025
-
What Medium Do Mechanical Waves Travel Through The Fastest
Mar 21, 2025
-
7 To The Power Of 7
Mar 21, 2025
-
Find The Area Inside The Oval Limacon
Mar 21, 2025
-
Write A Polynomial In Standard Form
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Why Lipids Are Not Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
