Why Does Ionisation Energy Decrease Down A Group
listenit
Mar 21, 2025 · 6 min read
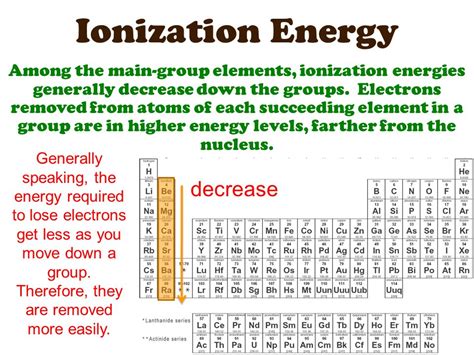
Table of Contents
Why Does Ionization Energy Decrease Down a Group? A Deep Dive into Periodic Trends
Ionization energy, a fundamental concept in chemistry, dictates the energy required to remove an electron from a gaseous atom or ion. Understanding its trends within the periodic table is crucial for predicting chemical behavior and reactivity. One of the most striking trends is the decrease in ionization energy down a group. This article will delve deep into the reasons behind this decrease, exploring the underlying physical principles and providing a comprehensive explanation.
The Basics: What is Ionization Energy?
Before exploring the trend, let's establish a clear understanding of ionization energy itself. It's defined as the minimum energy needed to remove the most loosely bound electron from a neutral gaseous atom. This process is represented by the equation:
X(g) + energy → X⁺(g) + e⁻
Where:
- X(g) represents a neutral gaseous atom of element X.
- X⁺(g) represents the gaseous ion formed after the removal of one electron.
- e⁻ represents the removed electron.
The ionization energy is usually expressed in kilojoules per mole (kJ/mol) or electron volts (eV). The first ionization energy refers to the removal of the first electron, the second ionization energy refers to the removal of the second electron, and so on. Each successive ionization energy is always higher than the preceding one because removing an electron from a positively charged ion requires more energy due to the increased electrostatic attraction.
The Downward Trend: Why Ionization Energy Decreases Down a Group
The consistent decrease in ionization energy as we move down a group in the periodic table is a direct consequence of several factors:
1. Increasing Atomic Radius: The Distance Factor
As we descend a group, the number of electron shells increases. This leads to a significant increase in the atomic radius – the distance between the nucleus and the outermost electrons. The further away the valence electrons are from the positively charged nucleus, the weaker the electrostatic force of attraction. This weaker attraction means less energy is required to remove an electron, hence the lower ionization energy.
Think of it like this: Imagine trying to pull a ball attached to a string. If the string is short, it requires more force. If the string is long, it requires less force. The atomic radius is analogous to the length of the string, and the electron is the ball.
2. Shielding Effect: The Electron Cloud's Influence
The increasing number of electron shells also enhances the shielding effect. Inner electrons shield the outer valence electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the valence electrons. A lower effective nuclear charge means a weaker attraction between the nucleus and the valence electrons, resulting in a lower ionization energy.
The inner electrons act like a buffer, reducing the pull of the nucleus on the outermost electrons. The more inner electrons there are (as you go down a group), the greater the shielding effect, and thus the easier it is to remove a valence electron.
3. Increased Number of Protons, but a Weaker Effective Nuclear Charge
While the number of protons in the nucleus increases down a group, the increase in shielding effect outweighs the increase in nuclear charge. The effective nuclear charge – the net positive charge experienced by the valence electrons – actually increases less dramatically than the number of protons. This relatively smaller increase in effective nuclear charge contributes to the lower ionization energy.
The added protons do increase the attraction, but the added electrons in the inner shells effectively shield the outer electrons, preventing the increase in attraction from being as significant as the increase in atomic radius.
Illustrative Examples: Comparing Ionization Energies
Let's consider Group 1 (alkali metals) to illustrate this trend:
- Lithium (Li): First ionization energy is relatively high because the valence electron is relatively close to the nucleus and experiences a strong effective nuclear charge.
- Sodium (Na): The first ionization energy is lower than lithium's because the valence electron is further from the nucleus and is shielded by the inner 2s and 2p electrons.
- Potassium (K): The first ionization energy is even lower than sodium's due to an even larger atomic radius and greater shielding effect from the additional inner shells.
This downward trend continues as we progress through the alkali metals, with the ionization energy consistently decreasing as we move from lithium to francium. This pattern repeats itself across other groups in the periodic table.
Exceptions and Nuances: Irregularities in the Trend
While the general trend of decreasing ionization energy down a group is well-established, there can be minor irregularities. These irregularities usually arise from subtle variations in electron configuration or interelectronic repulsions. For example, slight increases in ionization energy might be observed in certain periods due to the specific arrangement of electrons within subshells. However, these deviations are usually small and don't alter the overall downward trend.
Furthermore, the concept of ionization energy pertains to gaseous atoms. The presence of other atoms or molecules in a condensed phase (solid or liquid) can influence the energy required for ionization, adding complexity to the scenario.
Applications and Significance: Understanding Chemical Reactivity
The decrease in ionization energy down a group profoundly impacts the chemical reactivity of elements. Elements with low ionization energies readily lose electrons, forming positive ions. This makes them highly reactive, particularly in the case of alkali metals. For instance, the ease with which alkali metals lose their valence electrons contributes to their high reactivity and explains why they are rarely found in nature as uncombined elements.
Conversely, elements with high ionization energies tend to be less reactive, as they require significantly more energy to lose electrons. Understanding ionization energy trends, therefore, provides crucial insight into the reactivity and bonding behaviors of elements throughout the periodic table.
Conclusion: A Fundamental Periodic Trend
The decrease in ionization energy down a group is a cornerstone principle in understanding periodic trends and the chemical behavior of elements. The interplay between increasing atomic radius, enhanced shielding effect, and the relatively weaker increase in effective nuclear charge dictates this crucial pattern. This trend has far-reaching implications, impacting chemical reactivity, bonding, and the overall properties of elements. By grasping the reasons behind this trend, we can better understand and predict the behavior of atoms and molecules, leading to advancements in various fields of chemistry and beyond. Furthermore, appreciating this fundamental concept strengthens our understanding of the organization and principles underlying the periodic table itself. The decrease in ionization energy is not merely a trend to memorize but a consequence of fundamental physical laws governing atomic structure and behavior.
Latest Posts
Latest Posts
-
What Percent Is 1 Of 7
Mar 27, 2025
-
When Gas Exerts Pressure On Its Container The Pressure Is
Mar 27, 2025
-
X 3 2 X 1 2
Mar 27, 2025
-
60 Of What Number Is 20
Mar 27, 2025
-
Graph The Linear Equation X 4
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Why Does Ionisation Energy Decrease Down A Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
