Why Does Hot Water Dissolve Things Faster
listenit
Mar 28, 2025 · 5 min read
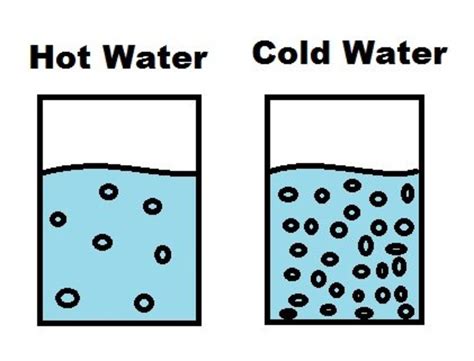
Table of Contents
Why Does Hot Water Dissolve Things Faster? A Deep Dive into the Science of Solubility
Hot water's superior dissolving power is a common observation, from making tea to cleaning dishes. But why exactly does it work better than cold water? The answer lies in the fascinating world of molecular motion, energy, and the very nature of solubility. This article delves into the scientific principles behind this phenomenon, exploring the impact of temperature on solubility, different types of solutes and solvents, and real-world applications.
The Role of Kinetic Energy and Molecular Motion
At the heart of this phenomenon lies kinetic energy. Molecules, the fundamental building blocks of matter, are constantly in motion. This motion increases with temperature. In cold water, molecules move slowly, colliding infrequently with solute particles. Think of it like a slow, sluggish dance.
Higher Temperature, Faster Dance
In hot water, however, the molecules possess significantly higher kinetic energy, leading to more frequent and forceful collisions. This is like turning on the music and encouraging a lively, energetic dance. These more frequent and energetic collisions are crucial for breaking apart the bonds holding solute particles together and allowing them to be surrounded and separated by water molecules – a process called solvation or dissolution.
Understanding Solubility: A Matter of Attraction
Solubility refers to the maximum amount of a substance (solute) that can dissolve in a given amount of solvent (like water) at a specific temperature and pressure. The solubility of a substance is determined by the interplay of several forces:
-
Intermolecular Forces: The attractive forces between molecules play a crucial role. Water molecules are polar, meaning they have a slightly positive end and a slightly negative end. This polarity allows them to effectively interact with and dissolve other polar substances, such as sugar and salt. Nonpolar substances, like oil, have weak intermolecular forces and tend to be insoluble in water.
-
Energy Changes: Dissolving a substance involves breaking the bonds within the solute and forming new bonds between the solute and solvent molecules. This process involves an energy change, sometimes releasing energy (exothermic) and other times requiring energy (endothermic). Heat aids in overcoming the energy barrier to dissolution.
Temperature's Impact on Solubility: A Detailed Analysis
The relationship between temperature and solubility is not always straightforward. It depends heavily on whether the dissolution process is exothermic or endothermic.
Exothermic Dissolution: Cooling Effect
In exothermic dissolution, the process of dissolving releases heat. Think of dissolving certain salts in water – the solution might become slightly cooler. In these cases, increasing temperature actually decreases solubility. The system seeks equilibrium, and by adding heat, it shifts the equilibrium toward less dissolved solute.
Endothermic Dissolution: Heating Advantage
For most solids dissolving in liquids (like sugar in water), the dissolution process is endothermic, meaning it absorbs heat. Increasing the temperature provides the extra energy required to break the bonds within the solid and allow the solute particles to be surrounded by solvent molecules. This is why hot water dissolves most solids faster than cold water.
Gases and Temperature: An Inverse Relationship
Gases exhibit a unique relationship with temperature. As temperature increases, the solubility of gases in liquids typically decreases. This is because the increased kinetic energy of gas molecules allows them to overcome the attractive forces holding them in solution, causing them to escape into the atmosphere. This is why opening a warm soda leads to more fizzing than opening a cold one.
Beyond Temperature: Factors Affecting Dissolution Rate
While temperature plays a dominant role, other factors also influence how quickly a substance dissolves:
-
Surface Area: Crushing or grinding a solid increases its surface area, exposing more solute particles to the solvent. This accelerates the dissolution process. Imagine dissolving a sugar cube versus granulated sugar – the latter dissolves much faster.
-
Agitation: Stirring or shaking a solution enhances the contact between the solute and solvent, promoting faster dissolution. This ensures constant replenishment of fresh solvent molecules around the solute particles.
-
Concentration: The concentration of the solute already in the solution affects the dissolution rate. A saturated solution (one that contains the maximum amount of solute) will not dissolve any more solute, regardless of temperature or agitation.
Real-World Applications: From Cooking to Chemistry
The principles governing the dissolution of substances in hot water find widespread applications in various fields:
-
Cooking: Hot water is crucial in extracting flavors and nutrients from ingredients, such as making tea, coffee, or broth. The higher temperature accelerates the dissolution of flavor compounds, resulting in a more flavorful and aromatic beverage or dish.
-
Cleaning: Hot water is more effective in dissolving dirt, grease, and other substances from surfaces. The increased kinetic energy of water molecules aids in breaking down these materials and carrying them away. This is why hot water is often preferred for washing dishes, laundry, and cleaning floors.
-
Industrial Processes: Many industrial processes rely on the solubility of substances in hot water for efficient extraction, purification, and chemical reactions. For instance, the production of certain chemicals involves dissolving raw materials in hot water to separate impurities.
-
Medicine: The solubility of drugs in hot water is a key factor in the formulation of oral medications. Hot water can help dissolve poorly soluble drugs, making them easier to absorb by the body.
Conclusion: Unraveling the Mysteries of Solubility
The enhanced dissolving power of hot water stems from the increased kinetic energy of water molecules, leading to more frequent and forceful collisions with solute particles. Understanding this fundamental principle allows us to manipulate the dissolution process, optimizing various applications, from simple household chores to complex industrial processes. The relationship between temperature and solubility, however, is nuanced, dependent on whether the dissolution process is exothermic or endothermic, and further influenced by other factors such as surface area, agitation, and concentration. The ability to effectively control and utilize the dissolving power of hot water remains a crucial aspect in many scientific and technological domains. By understanding the interplay of these factors, we can harness the power of hot water for a wide array of practical applications.
Latest Posts
Latest Posts
-
How To Subtract Negative And Positive Fractions
Mar 31, 2025
-
Find The Area Of A Triangle With Vertices
Mar 31, 2025
-
What Is At The Center Of Every Atom
Mar 31, 2025
-
6 As A Percentage Of 20
Mar 31, 2025
-
What Is The Name Of The Ionic Compound Cao
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Why Does Hot Water Dissolve Things Faster . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
