What Is At The Center Of Every Atom
listenit
Mar 31, 2025 · 7 min read
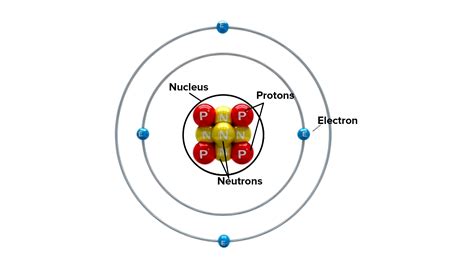
Table of Contents
What Lies at the Heart of Every Atom? A Deep Dive into the Atomic Nucleus
The atom. A word that conjures images of tiny, indivisible particles, the fundamental building blocks of everything around us. But the reality is far more complex and fascinating. While the ancient Greeks conceived of atoms as the smallest possible units of matter, modern science has revealed a world within the atom, a bustling subatomic realm dominated by the atomic nucleus. This article delves into the heart of every atom, exploring the composition, properties, and significance of the nucleus, from its fundamental particles to its role in shaping the universe.
Unveiling the Nucleus: Protons and Neutrons
At the atom's center lies the nucleus, a dense, positively charged region containing two types of particles: protons and neutrons. These particles, collectively known as nucleons, are bound together by an incredibly powerful force – the strong nuclear force. This force, significantly stronger than the electromagnetic force that repels the positively charged protons, is responsible for holding the nucleus together, preventing it from flying apart.
Protons: The Positive Charge Carriers
Protons are positively charged particles with a mass approximately 1836 times greater than that of an electron. The number of protons in an atom's nucleus, known as the atomic number, defines the element. For example, an atom with one proton is hydrogen, two protons is helium, and so on. This number is crucial because it dictates the atom's chemical properties and its place on the periodic table. The positive charge of the protons is what attracts the negatively charged electrons, forming the atom's structure.
Neutrons: The Nuclear Glue
Neutrons, as their name suggests, carry no electrical charge. Their mass is slightly larger than that of a proton. While not directly contributing to the atom's chemical properties, neutrons play a critical role in nuclear stability. They act as a buffer between the positively charged protons, helping to overcome the electromagnetic repulsion and maintain the integrity of the nucleus. The number of neutrons in an atom's nucleus is known as the neutron number. Atoms of the same element can have different numbers of neutrons, leading to isotopes.
Isotopes: Variations on a Theme
Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. This difference in neutron number can affect an atom's stability. Some isotopes are stable, meaning they remain unchanged over time. Others are unstable or radioactive, undergoing decay and emitting radiation in the process. This radioactive decay can involve the emission of alpha particles (helium nuclei), beta particles (electrons or positrons), or gamma rays (high-energy photons).
Radioactive Decay and its Applications
Radioactive decay is a fundamental process with various applications in diverse fields. In medicine, radioactive isotopes are used in diagnostic imaging (PET scans) and cancer treatment (radiotherapy). In archaeology and geology, radioactive dating techniques, such as carbon-14 dating, allow scientists to determine the age of ancient artifacts and geological formations. In industry, radioactive isotopes are used in various applications, including gauging the thickness of materials and sterilization of medical equipment.
Delving Deeper: The Subatomic Particles
The protons and neutrons themselves are not fundamental particles. They are composed of even smaller particles called quarks. Each proton and neutron consists of three quarks held together by the strong force.
Quarks: The Fundamental Building Blocks
There are six types of quarks: up, down, charm, strange, top, and bottom. Protons are made of two up quarks and one down quark, while neutrons are made of one up quark and two down quarks. The complex interactions between quarks and the strong force are governed by the theory of quantum chromodynamics (QCD), a cornerstone of the Standard Model of particle physics.
The Strong Nuclear Force: The Unsung Hero
The strong nuclear force is one of the four fundamental forces in nature, alongside the weak nuclear force, electromagnetism, and gravity. It's responsible for binding quarks together to form protons and neutrons and for holding the nucleons together within the nucleus. This force is extremely strong at short distances, but its influence diminishes rapidly as the distance increases. This short-range nature explains why the nucleus is so small compared to the overall size of the atom.
Understanding the Strong Force: A Complex Interaction
The strong force is mediated by particles called gluons. These gluons are constantly exchanged between quarks, creating the strong attractive force that binds them together. Understanding the dynamics of the strong force is a complex problem that continues to challenge physicists.
Nuclear Size and Density: A Tiny Giant
The nucleus is incredibly small, occupying only a tiny fraction of the atom's volume. However, it contains almost all of the atom's mass, making it incredibly dense. The density of the nucleus is so high that a teaspoonful of nuclear matter would weigh billions of tons! This extreme density is a consequence of the strong nuclear force packing nucleons tightly together.
Nuclear Reactions: Unleashing the Power Within
Nuclear reactions involve changes in the composition of an atom's nucleus. These reactions can release enormous amounts of energy, as seen in nuclear fission and fusion.
Nuclear Fission: Splitting the Atom
Nuclear fission is the process of splitting a heavy atomic nucleus into two or more lighter nuclei. This process releases a tremendous amount of energy, as demonstrated by the development of nuclear weapons and nuclear power plants. The energy released is due to the conversion of a small amount of mass into energy, according to Einstein's famous equation, E=mc².
Nuclear Fusion: Powering the Stars
Nuclear fusion is the process of combining two or more lighter nuclei to form a heavier nucleus. This process also releases vast amounts of energy, even more than fission. Fusion is the primary energy source of stars, including our Sun. The immense pressure and temperature within stars are needed to overcome the electrostatic repulsion between the positively charged nuclei and allow them to fuse.
The Nucleus and the Periodic Table
The atomic nucleus plays a pivotal role in shaping the periodic table of elements. The number of protons in an atom's nucleus determines its atomic number and, consequently, its identity and chemical properties. Elements are arranged in the periodic table according to their atomic numbers, reflecting their electronic configurations and resulting chemical behavior. The properties of elements change predictably as you move across and down the table, thanks to the systematic increase in the number of protons in the nucleus.
Nuclear Physics: Ongoing Research and Future Directions
Nuclear physics is a field of ongoing research. Scientists are continually seeking to better understand the properties of the nucleus, the strong nuclear force, and nuclear reactions. This research has led to advancements in numerous fields, from medicine and energy to materials science and fundamental physics. Current research focuses on various aspects, including:
- Nuclear Structure: Scientists are studying the detailed structure of nuclei, trying to understand how nucleons arrange themselves and how this arrangement affects nuclear properties.
- Nuclear Reactions: Research is ongoing to explore new types of nuclear reactions and their potential applications. This includes studying reactions relevant to nuclear energy and the synthesis of new elements.
- Nuclear Astrophysics: Nuclear processes are crucial for understanding stellar evolution and the formation of elements in the universe. This interdisciplinary field combines nuclear physics with astronomy and astrophysics.
- Fundamental Interactions: Studies are conducted to refine our understanding of the strong and weak nuclear forces and their connections to other fundamental interactions.
Conclusion: A Universe Within
The atomic nucleus, a tiny powerhouse at the heart of every atom, holds a universe of complexity and wonder. From the fundamental particles that compose it to the powerful forces that govern its behavior, the nucleus is a testament to the intricate workings of nature. Its study has not only expanded our understanding of the universe but also given rise to technologies that are transforming society. As research continues, we can expect further discoveries and deeper insights into the profound secrets held within this infinitesimally small yet immensely significant component of all matter. The journey to unraveling the mysteries of the atomic nucleus is far from over, promising exciting discoveries and advancements in the years to come.
Latest Posts
Latest Posts
-
All Atoms Of The Same Element Have
Apr 02, 2025
-
Animals That Feed Exclusively On Plants Are Called
Apr 02, 2025
-
What Is The Equivalent Fraction Of 3 4
Apr 02, 2025
-
Least Common Multiple Of 12 And 4
Apr 02, 2025
-
What Does 2 1 2 Mean
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is At The Center Of Every Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
